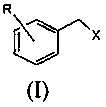
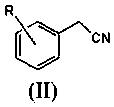
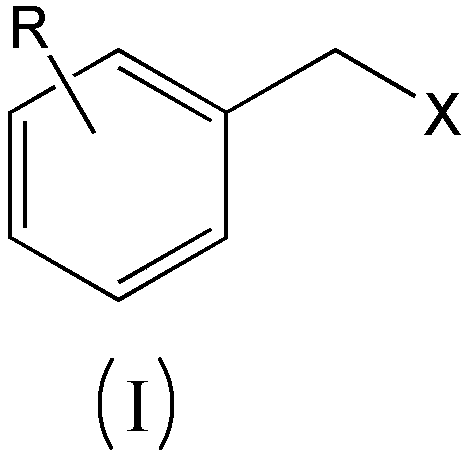
A kind of preparation method of substituted benzyl nitrile
A phenylacetonitrile reaction technology, applied in the field of organic synthesis, can solve the problems of low reaction efficiency, large floor space, unfavorable operation, etc., and achieve the effects of simple process route, less equipment and low environmental protection treatment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]Simultaneously the mol ratio of accurate metering is: benzyl chloride: benzyl chloride and hydrogen cyanide gas that hydrogen cyanide is 1.6: 1.0 are respectively preheated to 155.0 ℃, then enter the microchannel reactor after input mixer mix simultaneously, ensure The molar ratio of the two is always stable at 1.6:1.0, and the nucleophilic substitution reaction is carried out at 180.0°C and 1.5MPa pressure to generate substituted phenylacetonitrile and hydrogen halide. The reaction residence time is 40s, and then the reaction material is cooled to 85°C to quench the reaction. Then introduce the material into the water washing tower to wash off the hydrogen chloride generated by the reaction, let it stand for liquid separation, add 10% sodium bicarbonate solution to the organic phase to neutralize to a pH value of 6.8, let it stand for liquid separation, add water to wash once, and let it stand for further separation. liquid. Then rectify under the pressure of 300Pa to o...
Embodiment 2
[0042] At the same time, the accurately metered molar ratio of o-chlorobenzyl chloride: hydrogen cyanide is 1.8:1.0, and the o-chlorobenzyl chloride and hydrogen cyanide gas are preheated to 165.0°C respectively, and then input into the mixer at the same time to mix and then enter the microchannel Reactor, to ensure that the molar ratio of the two is constantly stable at 1.8:1.0, nucleophilic substitution reaction is carried out at 175.0°C and 1.7MPa pressure to generate o-chlorophenylacetonitrile and hydrogen chloride, the reaction residence time is 90s, and then the reaction material is cooled to 90°C, Quench the reaction, then introduce the material into the water washing tower to wash away the hydrogen chloride generated by the reaction, let it stand for liquid separation, add 5% sodium carbonate solution to the organic phase to neutralize to pH 7.2, let it stand for liquid separation, add water to wash once, and let it stand for liquid separation. Set aside for redistribut...
Embodiment 3
[0044] At the same time, the accurately measured molar ratio is: o-methylbenzyl chloride: hydrogen cyanide 1.8:1.0 o-methylbenzyl chloride and hydrogen cyanide gas were preheated to 175.0 ° C, and then simultaneously input into the mixer and mixed into the Microchannel reactor, to ensure that the molar ratio of the two is always stable at 1.8:1.0, the nucleophilic substitution reaction is carried out at 190.0 ° C and 1.8 MPa pressure to generate o-toluene acetonitrile and hydrogen chloride, the reaction residence time is 120s, and then the reaction material is cooled to 80°C, quench the reaction, then introduce the material into the water washing tower to wash away the hydrogen chloride generated by the reaction, let it stand for liquid separation, add 5% calcium hydroxide solution to the organic phase to neutralize to pH 6.5, let it stand for liquid separation, and then add water Wash once, let stand and then separate liquid. Then rectify under the pressure of 260Pa to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com