Test paper for testing chorionic gonadotropin in human saliva and preparation method thereof
A chorionic gonadotropic and human detection technology, applied in biological testing, measuring devices, material inspection products, etc., can solve problems such as interference sensitivity, achieve high sensitivity of false positive rate, improve good labeling, and enhance the effect of detection signals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] Another aspect of the present invention provides the preparation method of the first aspect test paper, comprising the steps of:
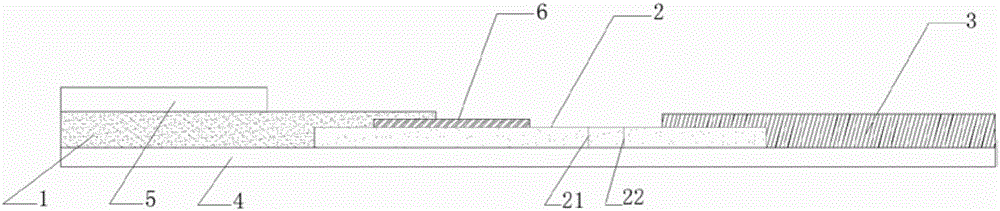
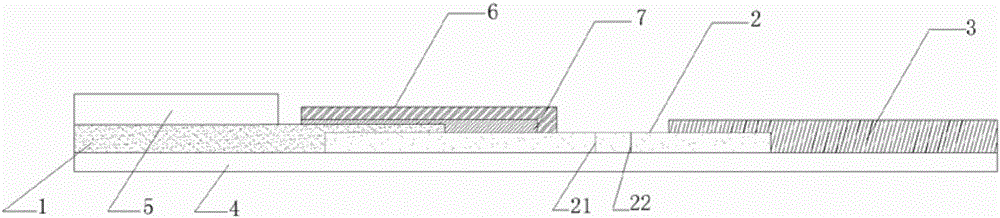
[0052] S1: Preparation of sample pads
[0053] Preparation of the first sample pad 1: place the cut glass fiber membrane, or non-woven fabric, or filter test paper in the treatment liquid of the first sample pad 1, soak at room temperature, dry, and set aside;
[0054] Further, the preparation method of the first sample pad 1 treatment solution is as follows: weigh 0.5g±0.05g BSA into a beaker, add 70ml of 10mM pH8.4 borate buffer solution to dissolve, add Tween-20, mix well, Constant volume, filter membrane and set aside.
[0055] The preparation of the second sample pad 5: choose filter test paper with a pore size of 500nm-1000um, or glass fiber membrane, or non-woven fabric, or water-absorbing filter paper, soak and dry in a hydroxyapatite liquid with a concentration of 0.1-100mg / ml at room temperature dry, spare;
[0056] Further, the...
Embodiment 1
[0069] Preparation of human salivary chorionic gonadotropin (HCG) rapid immunodiagnostic test paper
[0070] 1. The required raw materials are as follows:
[0071] 1.1 Reagents
[0072] Anti-α-HCG and β-HCG antibodies, cellulose nanoparticles (cellulose nanoparticles produced by Asahi Kasei, diameter 340±20mm), casein, hydroxyapatite, trehalose, NaCl, Na2HPO4·12H2O, NaH2PO4· 2H2O, boric acid, borax, Tween-20, Triton X-100, sucrose, ultrapure water, etc.;
[0073] 1.2 Consumables
[0074] Nitrocellulose membrane (NC membrane), 8975 glass fiber, GF-06 glass fiber, absorbent pad, bottom plate 4, volumetric flask, Erlenmeyer flask, EP tube, pipette tip;
[0075] 1.3 Solution preparation
[0076] Preparation of PBS buffer solution: Weigh 1.615-2g of Na2HPO4·12H2O, 0.225-5g of NaH2PO4·2H2O, and 4.0-5.0g of NaCl into a beaker, add 400ml of ultrapure water to dissolve to 500ml, filter through a 0.22μm filter membrane and set aside;
[0077] Preparation of 0.2M boric acid solution...
Embodiment 2
[0101] Sensitivity test of human salivary chorionic gonadotropin (HCG) rapid immunodiagnostic test strip
[0102] 1. Saliva collection: collect by saliva swabbing method, collect saliva and put it into saliva diluent, and rub both sides of the collection stick on the tube wall filled with saliva diluent for 5-6 times.
[0103] 2. Spiked saliva: Take 98 μL of saliva in the microwell, add 2.5 μL m IU / ml HCG standard to get the saliva spiked with 25 mIU / ml; take 98 μL and 95 μL of saliva in the microwell, add 2 μL and 5 μL 100mIU / ml HCG standard can be spiked with 2mIU / ml, 5mIU / ml saliva;
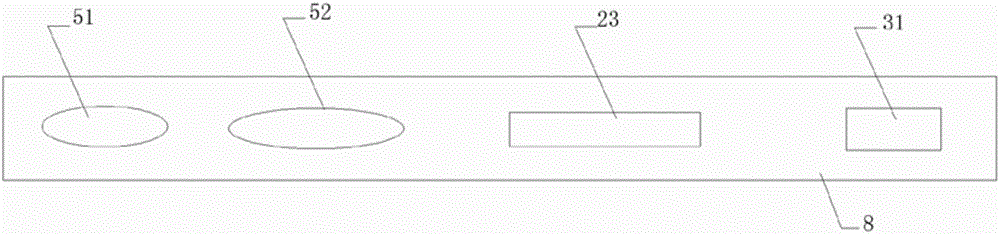
[0104] 3. Test: Put the dual-channel test strip on the table, take 50 μL of saliva and drop it on the second sample pad 5, and let it stand at room temperature for 1-5 minutes. After the color of the suction pad 3 changes, take 50 μL of LPBS buffer solution Put it on the binding pad 6 drop by drop, let it stand at room temperature for 5 minutes, observe the results, the sensitivity of saliva ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com