Method used for high efficiency adsorbing of heavy metals with functionalized magnetic material rich in thio amino groups
A technology that is rich in thioamine groups and adsorbs heavy metals. It is applied in chemical instruments and methods, alkali metal compounds, and adsorption of water/sewage treatment. It can solve the problems of few adsorption sites, low density of amino functional groups, adsorption capacity and adsorption Low rate and other problems, to achieve the effect of simple method, increased functional group density, fast and efficient adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
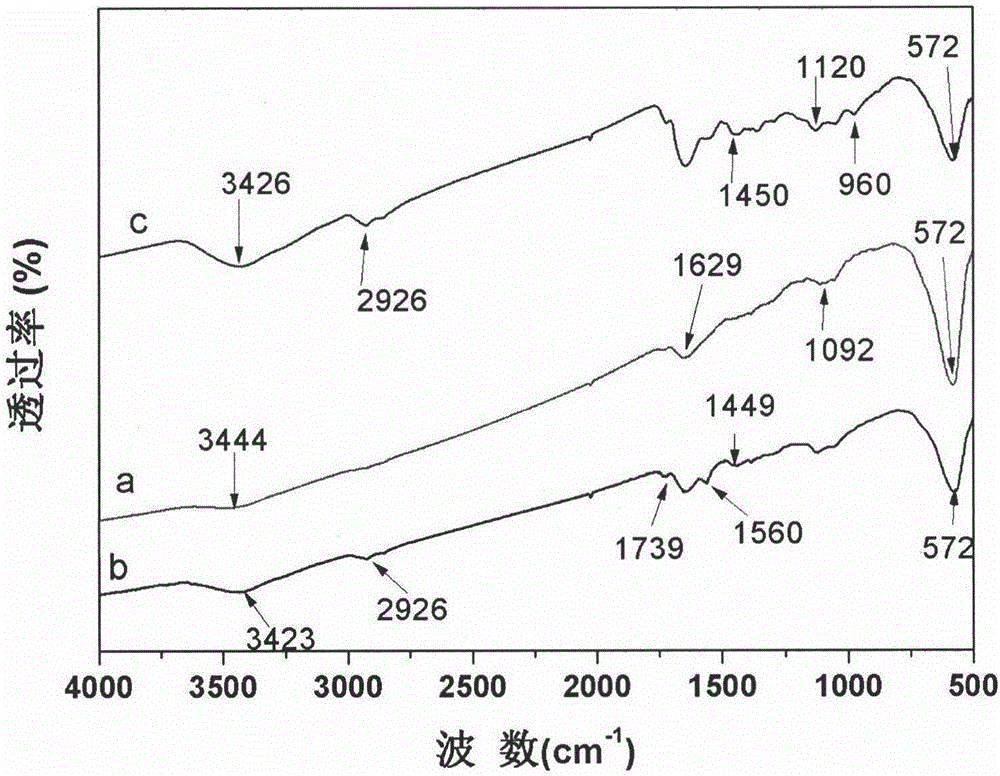
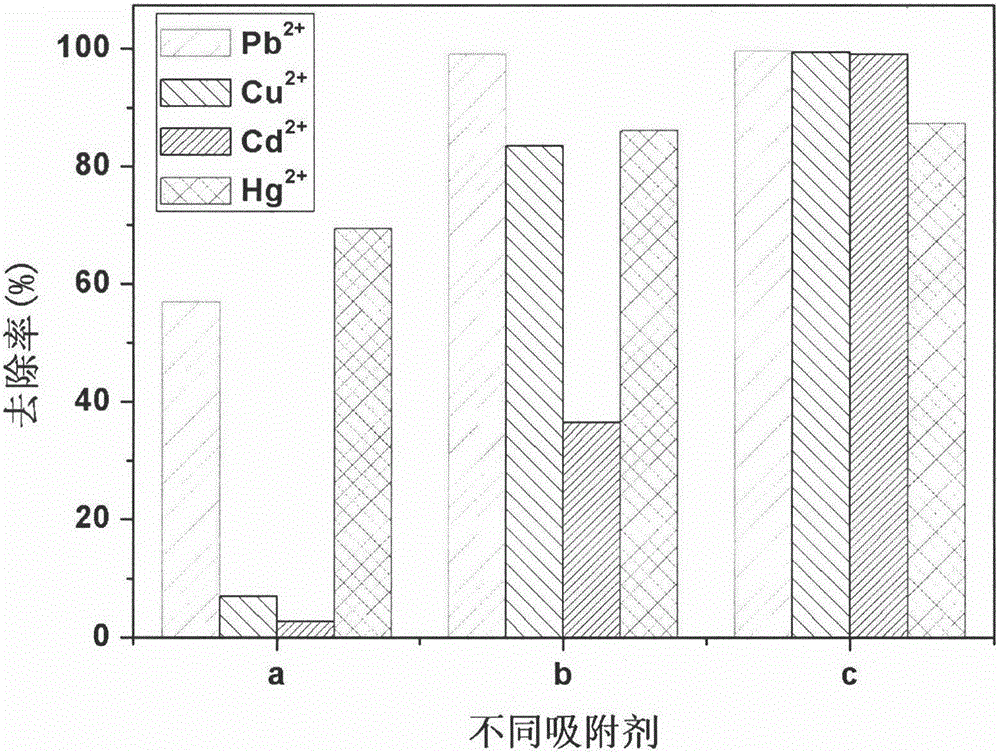
Image
Examples
Embodiment 1
[0026] a. Preparation of functionalized magnetic materials rich in thioamine groups
[0027] 1) Preparation of amine-terminated hyperbranched polymer: Dissolve 20.63g of diethylenetriamine in 25mL of methanol, then dropwise add 17.22g of methyl acrylate, react at room temperature for 10h, distill out the methanol in the system under reduced pressure, and then The reaction was continued at 60°C for 1 hour under vacuum, and the temperature of the oil bath was raised to 100-150°C for 8.5 hours. Add methanol to dissolve, and the reaction mixture is precipitated by ether, washed by suction filtration, and dried in vacuo to obtain a light yellow sticky solid;
[0028] 2) Preparation of amine-functionalized mesoporous magnetic material: 2.0 g of anhydrous FeCl 3 Dissolve 4.0 g of sodium acetate in 60 mL of ethylene glycol, stir and sonicate for about 1 h, and then add 20 mL of ethanolamine to react for 2.5 h under reflux at 40°C. The resulting mixture was reacted in an autoclave at...
Embodiment 2
[0034] a. Preparation of functionalized magnetic materials rich in thioamine groups
[0035] 1) Preparation of amine-terminated hyperbranched polymer: Dissolve 12.02g of ethylenediamine in 25mL of methanol, then dropwise add 17.22g of methyl acrylate, react at room temperature for 10h, distill out the methanol in the system under reduced pressure, and then vacuum The reaction was continued at 60°C for 1h, and the temperature of the oil bath was raised to 125°C for 8.5h. Add methanol to dissolve, and the reaction mixture is precipitated by ether, washed by suction filtration, and dried in vacuo to obtain a light yellow sticky solid;
[0036] 2) Preparation of amine-functionalized mesoporous magnetic material: 2.0 g of anhydrous FeCl 3 Dissolve 4.0 g of sodium acetate in 60 mL of ethylene glycol, stir and sonicate for about 1 h, and then add 20 mL of ethylenediamine to react for 2.5 h under reflux at 40°C. The resulting mixture was reacted in an autoclave at 200°C for 10 hours...
Embodiment 3
[0042] a. Preparation of functionalized magnetic materials rich in thioamine groups
[0043] 1) Preparation of amine-terminated hyperbranched polymer: Dissolve 29.29g of tetraethylenepentamine in 25mL of methanol, then dropwise add 22.83g of ethyl acrylate, react at room temperature for 10h, distill out the methanol in the system under reduced pressure, and then The reaction was continued at 60°C for 1 h under vacuum, and the temperature of the oil bath was raised to 125°C for 8.5 h. Add methanol to dissolve, and the reaction mixture is precipitated by ether, washed by suction filtration, and dried in vacuo to obtain a light yellow sticky solid;
[0044] 2) Preparation of amine-functionalized mesoporous magnetic material: 2.0 g of anhydrous FeCl 3 Dissolve 4.0 g of sodium acetate in 60 mL of ethylene glycol, stir and sonicate for about 1 h, and then add 20 mL of diethylenetriamine under reflux at 40°C for 2.5 h. The resulting mixture was reacted in an autoclave at 200°C for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com