Method for removing iron ions in trivalent chromium acid solution
An acid solution and trivalent chromium technology, applied in the fields of efficient removal of iron ions in trivalent chromium acid solution, iron removal agent circulation and high-value utilization of iron, can solve the problem of high iron removal cost and reduce iron removal agent consumption. , to achieve the effect of high-value utilization and short process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
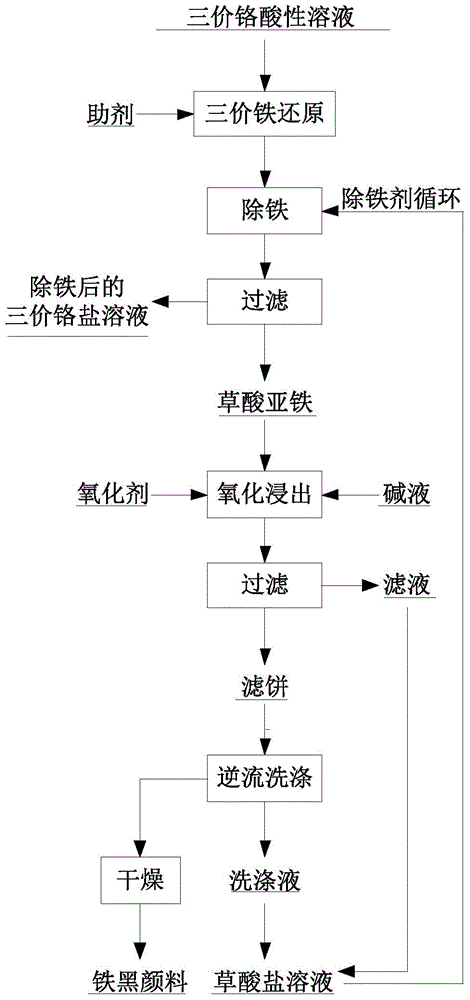
[0041] Add a theoretical amount of 105% hydrazine hydrate solution to the high-carbon ferrochrome acid hydrolysis solution, add sodium oxalate solution to the reaction solution after the reaction, adjust the solution to between 2.5 and 3.0, filter the reaction solution, and the filtrate is used to produce chromium Salt, obtained ferrous oxalate its main chemical composition w(FeC 2 o 4 2H 2 O)=94.8%, and other impurities are trace amounts of Mn, Ni, Co, etc.
[0042] Put the ferrous oxalate sample and ammonia water with a mass fraction of 27% in a three-necked flask, the reaction temperature is 60°C, and the liquid-solid ratio (L / kg) is maintained at 2:1, feed oxygen and start timing, and stop stirring after 2 hours of reaction , maintain the filtration temperature of 60°C to filter the reaction solution, after the filtration, wash the filter cake twice with clean water, and dry the filtered filter cake in an oven at 100°C to become iron oxide black, and combine the leaching...
Embodiment 2
[0044] Add a theoretical amount of 110% sodium metabisulfite to the vanadium-chromium slag sulfuric acid acid hydrolysis solution, add potassium oxalate solution to the reaction solution after the reaction, adjust the concentration of the solution to 2.5-3.0, filter the reaction solution, and the filtrate is used to produce chromium salt , the main chemical composition of the obtained ferrous oxalate w(FeC 2 o 4 2H 2 O)=95.1%, and other impurities are trace amounts of Mn, Ni, Co, etc.
[0045] Place the ferrous oxalate sample and 10% sodium hydroxide solution in a three-necked flask, the reaction temperature is 60°C, the liquid-solid ratio (L / kg) is maintained at 3:1, air is introduced and timing is started, and the reaction is 1.5 Stop stirring after h, maintain the filtration temperature of 70°C to filter the reaction solution, and wash the filter cake with clear water 3 times in countercurrent after the filtration, and the filter cake after filtration is iron oxide black ...
Embodiment 3
[0047] Add a theoretical amount of 110% vitamin C to the chromite acid hydrolysis solution, add ammonium oxalate solution to the reaction solution after the reaction, adjust the concentration of the solution to between 2.5 and 3.0, filter the reaction solution, and the filtrate is used to produce chromium salt. Its main chemical composition w(FeC 2 o 4 2H 2 O)=96.1%, and other impurities are trace amounts of Mn, Ni, Co, etc.
[0048] Place the ferrous oxalate sample and potassium hydroxide solution with a mass fraction of 40% in a three-necked flask, the reaction temperature is 80°C, and the liquid-solid ratio (L / kg) is maintained at 4:1, adding sodium peroxide solution and starting timing, After reacting for 2.2 hours, stop stirring, maintain the filtration temperature at 80°C to filter the reaction solution, and wash the filter cake with clean water for 4 times in countercurrent after filtration, and dry the filtered filter cake in an oven at 80°C to become iron oxide blac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com