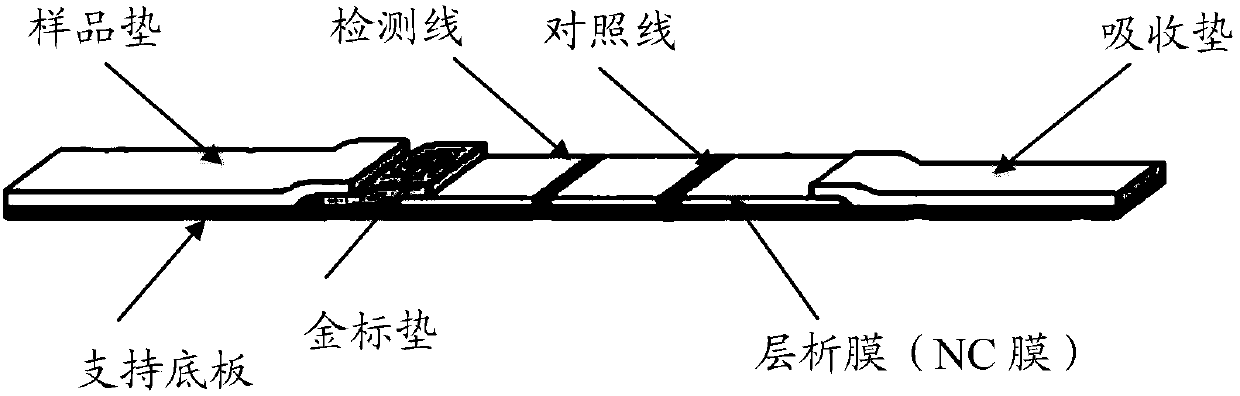
Anti-fluoroquinolone drug cluster-specific monoclonal antibody hybridoma cell line and the monoclonal antibody produced by it and its application
A hybridoma cell line and fluoroquinolone technology, applied in the biological field, can solve the problems of false negative detection accuracy, false positive detection specificity, low detection limit, etc., and achieve strong enrichment, strong versatility, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
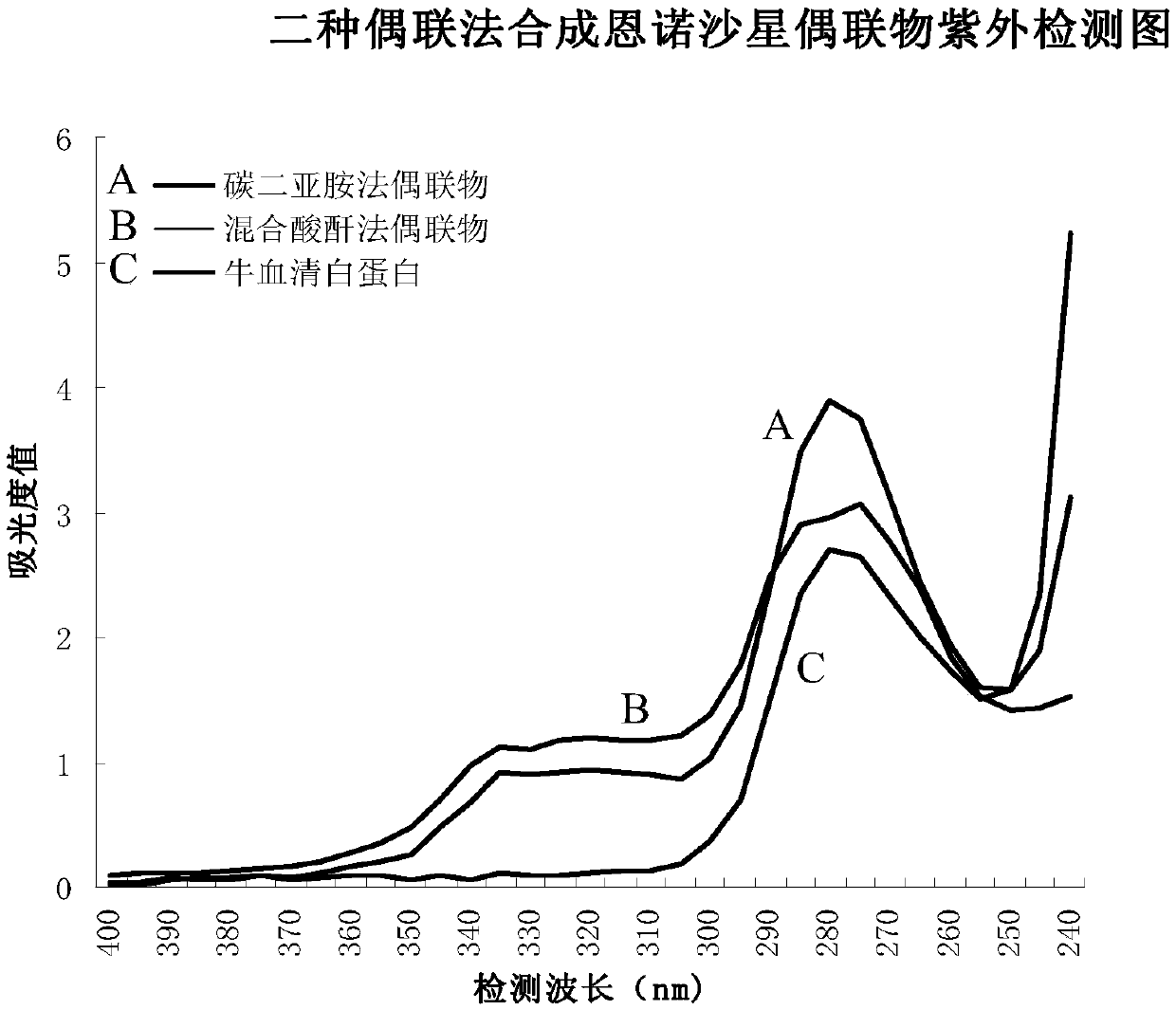
[0056] In the preparation of the immunogold standard card in the present invention, the enrofloxacin standard sample is preferably coupled with high-purity cationized bovine serum albumin (cBSA) by carbodiimide coupling method or mixed anhydride method. The conjugate is prepared as a test line (T line) for the coated antigen. The two methods are as follows:
[0057] 1. Carbodiimide coupling method
[0058] First, ethylenediamine (EDA) and bovine serum albumin were mixed and dissolved in 0.01M PBS (pH7.0) solution, and under the action of chemical coupling agent-dicycloethylcarbodiimide (EDC), the bovine serum was blocked. The -COOH residue in the albumin molecule is removed by dialysis to remove excess EDA and EDC, and cationized bovine serum albumin (cBSA) is obtained as a protein carrier.
[0059]Dissolve the standard sample of enrofloxacin (EF) in N,N-dimethylformamide (DMF), add N-hydroxysuccinimide (NHS), and react overnight in EDC in the dark at room temperature to form...
Embodiment 1
[0069] Example 1: In vivo induction of monoclonal antibody samples of the present invention
[0070] 1. Recovery and proliferation of hybridoma cell line -11F10
[0071] Take the hybridoma cell line-11F10 cryopreservation tube from the liquid nitrogen tank, melt it rapidly in a water bath at 37°C, centrifuge at 600r / min for 5min, discard the supernatant, add fresh 15% FBS / RPMI-1640 culture medium to suspend the cells, and supplement each After adding the above-mentioned culture solution to 5ml, plant it in a 50ml cell culture bottle and culture it in a carbon dioxide incubator. After the cells grow to a density of 30%, the medium is replaced halfway. Passage once every 2-3 days according to 1:3-4.
[0072] 2. In vivo induction of monoclonal antibody protein and ascites acquisition
[0073] 7-10 days before planting hybridoma cells in vivo, 12 8-week-old male BALB / c mice were injected intraperitoneally with pristane at 0.5ml / mouse, and carefully reared for later use.
[0074...
Embodiment 2
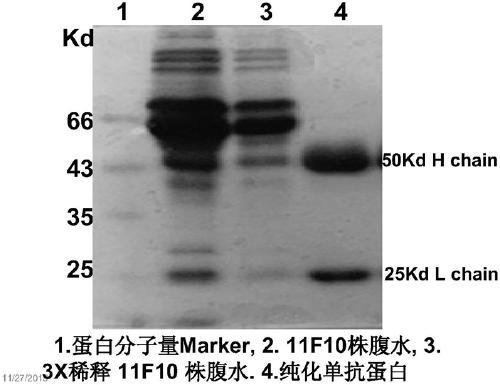
[0077] Embodiment 2: Purification of the monoclonal antibody protein of the present invention
[0078] Pre-cool 0.01M PBS (pH 8.0) 4× diluted ascitic fluid in an ice-water bath as a preparation solution. According to 50mg of total protein: 1ml Protein G Resin, fill the gel in a small column for chromatography (10ml), equilibrate the column with 50× pre-cooled 0.01M PBS (pH 8.0) of the column bed volume, and prepare the solution at room temperature Filter five times at the natural flow rate of the Protein G Resin column, so that the monoclonal antibody protein in the ascitic fluid can fully specifically bind to protein G, and then fully rinse with pre-cooled 0.01M PBS (pH8.0) solution 50 times the volume of the column bed Chromatographic column to remove foreign proteins adhering to the chromatographic gel.
[0079] Pre-add 100μl 1M Tris-Cl (pH 9.6) to each 1.5ml EP tube, add ice-cold 0.1M Glycine-HCl (pH 2.7) to the above-mentioned treatment column to elute, and collect the f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com