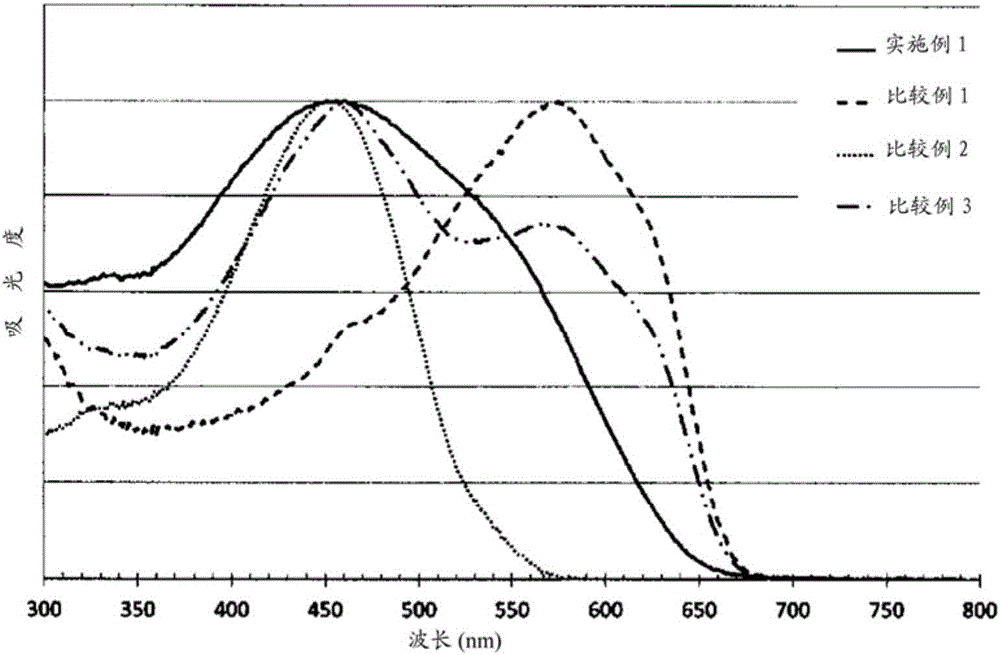
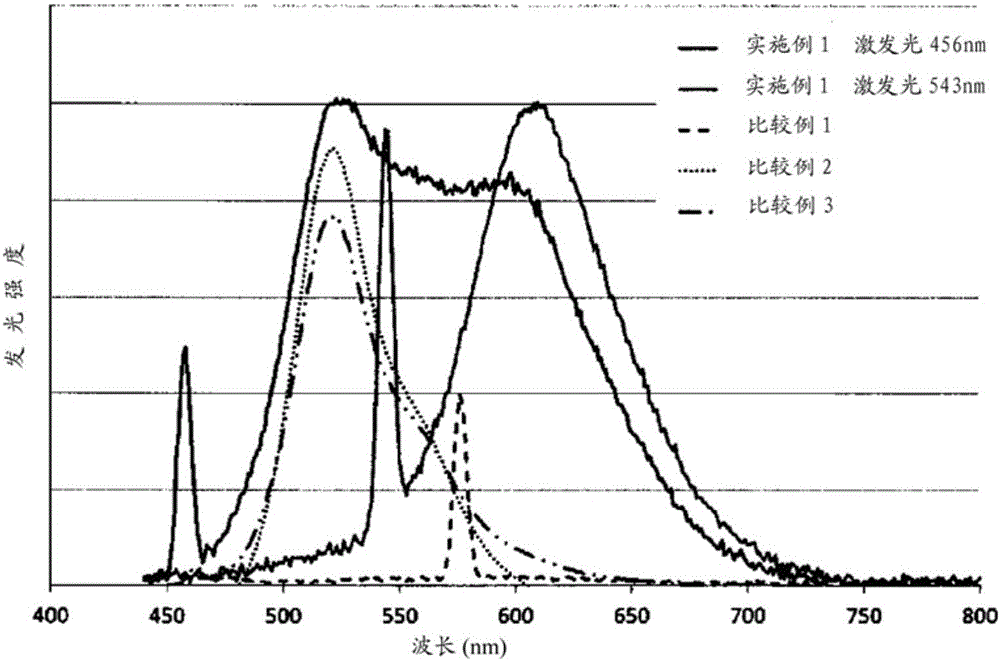
Organic heteropolymer and method for manufacturing same
A heteropolymer and organic technology, applied in the field of organic heteropolymers, can solve problems such as the limitation of the utilization of electronic devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0206] [chemical formula 20]
[0207]
[0208] (In the formula, R represents 2-ethylhexyl, and x and y represent the content ratio (molar ratio) of each structural unit, which is x:y=0.44:0.56.)
[0209] Under an argon atmosphere, 1,4-diethynyl-2,5-bis(2-ethylhexyloxy)benzene (0.191g, 0.500mmol) and tetraisopropoxytitanium (Ti(OPr i ) 4 ) (0.198g, 0.700mmol) was dissolved in cyclopentyl methyl ether (20ml), and while the solution was stirred at -78°C, isopropylmagnesium chloride was further added ( i PrMgCl) in diethyl ether (1.0 N, 1.25 ml, 1.25 mmol). Thereafter, the temperature was slowly raised to -50°C and stirred for 12 hours. At this temperature, dichlorophenylphosphine (0.053 g, 0.300 mmol) and disulfur dichloride (0.041 g, 0.300 mmol) were added step by step, Slowly warm to room temperature and further stir for 12 hours. Water was added to the obtained reaction solution, extracted with chloroform, and reprecipitated with hexane to obtain a red polymer represented...
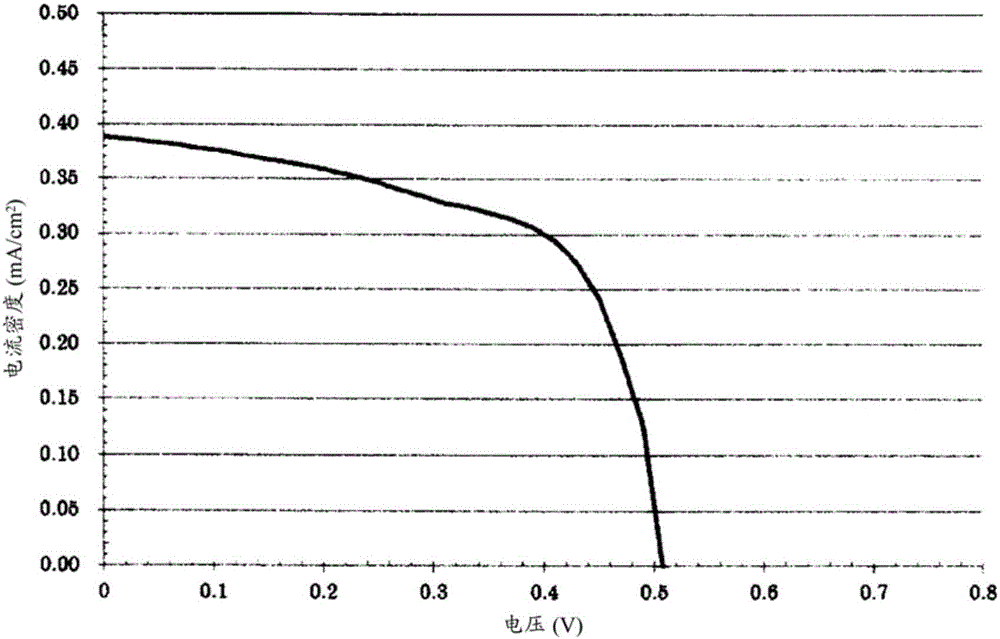
Embodiment 2
[0229] On FTO glass (manufactured by Astellatech Co., Ltd., model FTB) cleaned with acetone, a titanium oxide paste ("Ti-Nanoxide T / SP" manufactured by SOLARONIX Co., Ltd.) was formed into a 4 mm film with a thickness of 10 μm by the screen printing method The square was dried at 100° C. using a hot plate, and then baked at 500° C. for 1 hour to obtain a titanium oxide electrode.
[0230] The polymer obtained in Example 1 was dissolved in THF to prepare a 0.1% by weight solution. The aforementioned titanium oxide electrode was immersed in the solution, and left to stand at room temperature for 24 hours to allow the polymer obtained in Example 1 to be adsorbed on the surface of titanium oxide. After the adsorption, the titanium oxide electrode was taken out from the solution, washed with THF and dried to obtain a polymer-adsorbed titanium oxide electrode. As the counter electrode of this polymer-adsorbed titanium oxide electrode, a platinum thin film (thickness 0.003 μm) was f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com