Organic electroluminescent device and producing method thereof
An electroluminescent device, organic technology, applied in the direction of electroluminescent light sources, chemical instruments and methods, electric light sources, etc., can solve problems such as difficult purification, achieve firm adhesion, improve thermal stability and film-forming performance, and high thermal stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0044] The organic electroluminescence material of the present invention is prepared by a synthesis method in an organic solvent. The steps adopted include two parts: one is the synthesis of the corresponding tridentate ligand, and the other is the synthesis of the target complex.
[0045] The synthesis method of the related tridentate ligand in the first step adopts a Schiff base (Schiff base) synthesis method. The specific process is to directly mix and heat the aromatic aldehyde containing the o-hydroxyl in the stoichiometric ratio and the aromatic amine containing the o-hydroxyl in the solid phase, or heat and reflux the reaction in an alcohol solvent to obtain the crude product of the corresponding Schiffer base. Then by the method of recrystallization in organic solvent, it is easy to obtain the product with high purity.
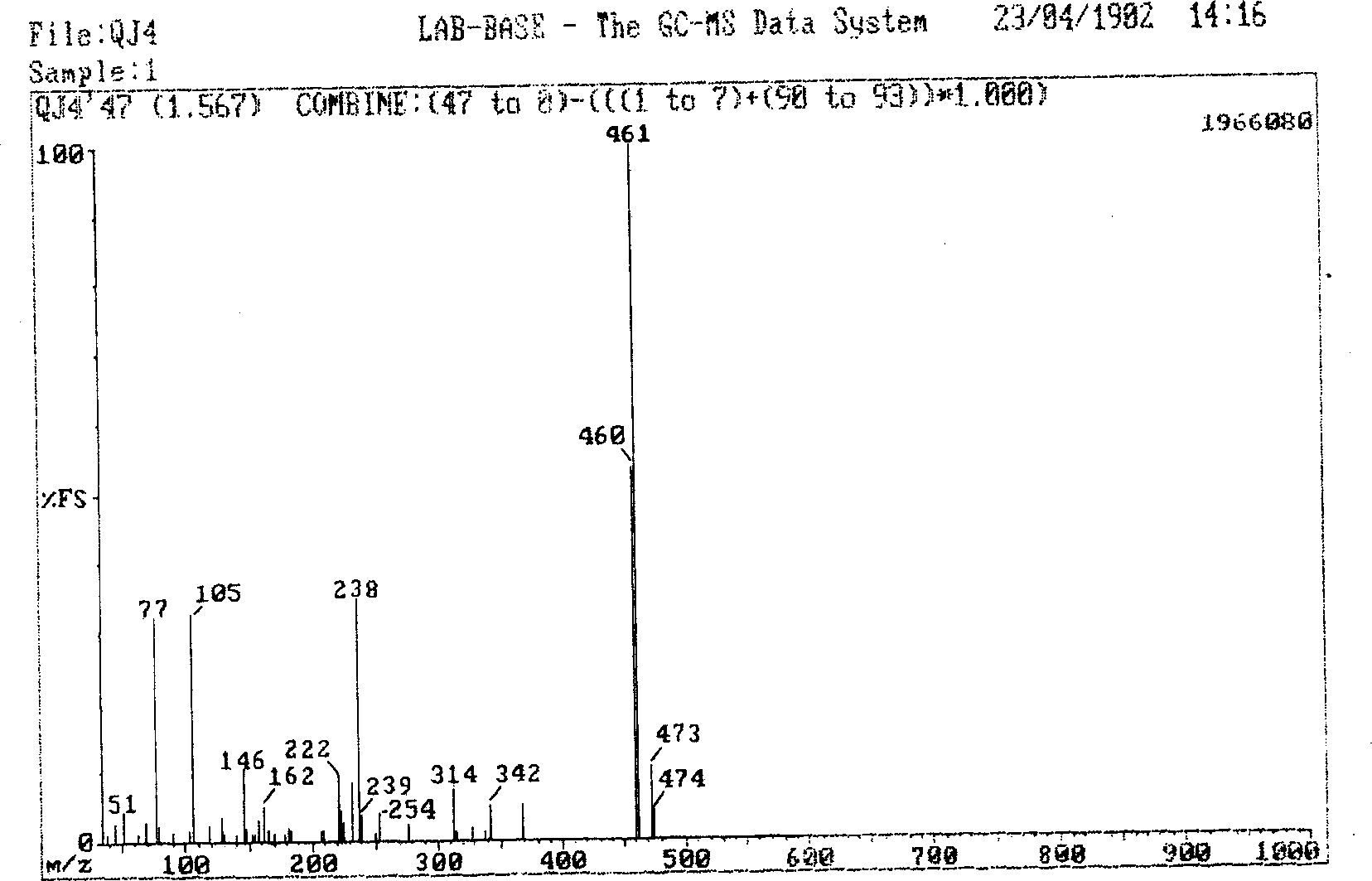
[0046] The synthesis of the target compound in the second step adopts inorganic salts of trivalent metals (such as gallium, aluminum, indium, iridium...
Embodiment 1
[0047] Embodiment 1: the synthesis of compound 1
[0048] Synthesis of ligand salicylaldehyde ortho-aminophenol:
[0049] Weigh 1.22 grams (0.01 mol) of salicylaldehyde and put it into a reaction bottle, add 1.09 grams (0.01 mol) of equimolar o-aminophenol, and heat directly, that is, a red substance will be produced. After the reaction is completed, use ethanol to recrystallize to obtain The needle-like product, salicylaldehyde-o-aminophenol, was obtained in 85% yield.
[0050] Synthesis of compound 1:
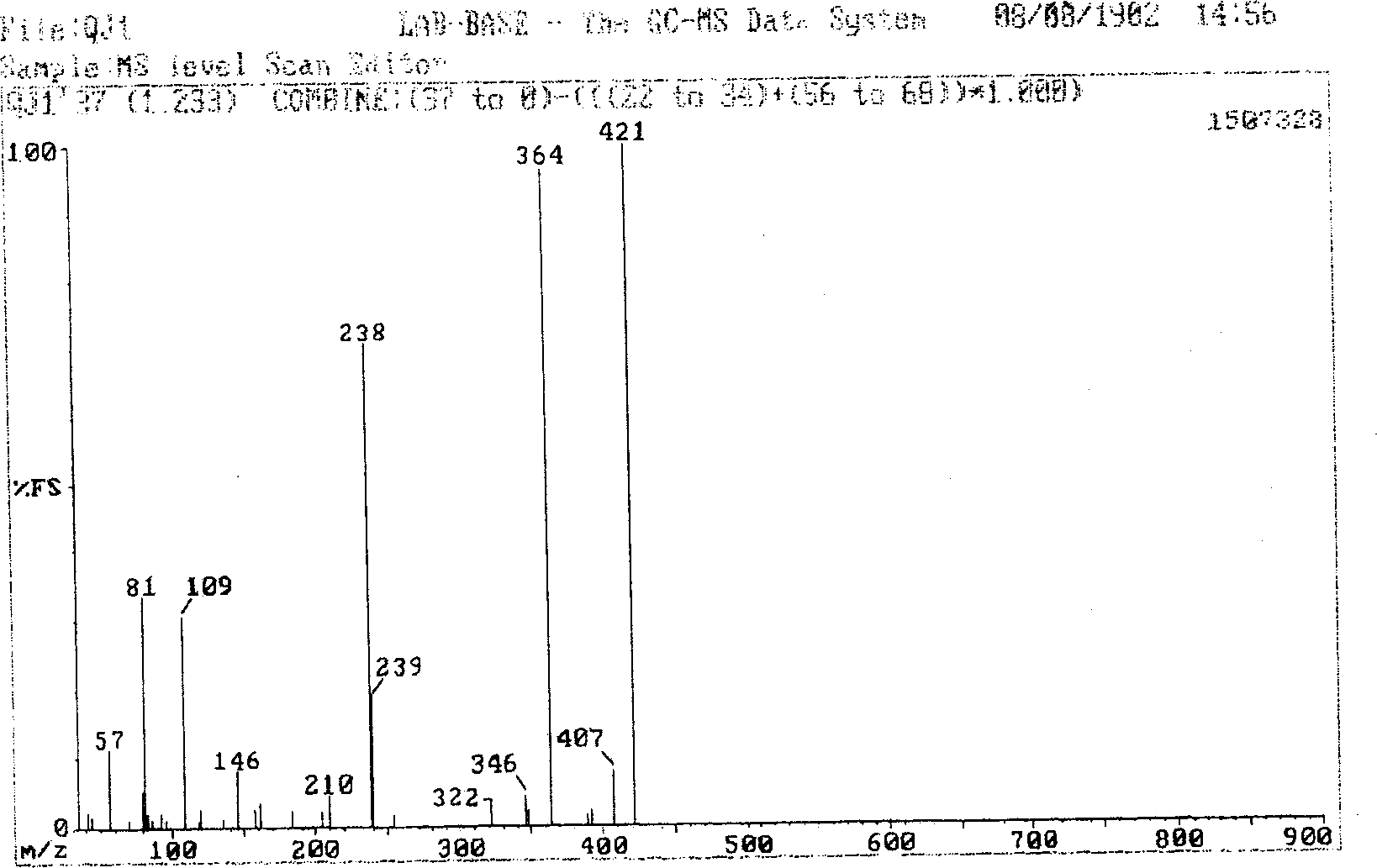
[0051] In a three-neck flask equipped with a stirring device, add 20 milliliters of 0.88 grams (0.005 moles) of anhydrous aluminum trichloride in ethanol. Completely dissolve 0.5 g (0.005 mol, 0.5 ml) of acetylacetone in a mixed solution of 5 ml of ethanol and 0.43 g (0.005 mol) of hexahydropyridine, slowly drop this solution into the above solution while stirring, and continue to Stir for 5 minutes. 1.065 grams (0.005 moles) of salicylaldehyde o-aminophenol was completel...
Embodiment 2
[0053] Embodiment 2: the synthesis of compound 2
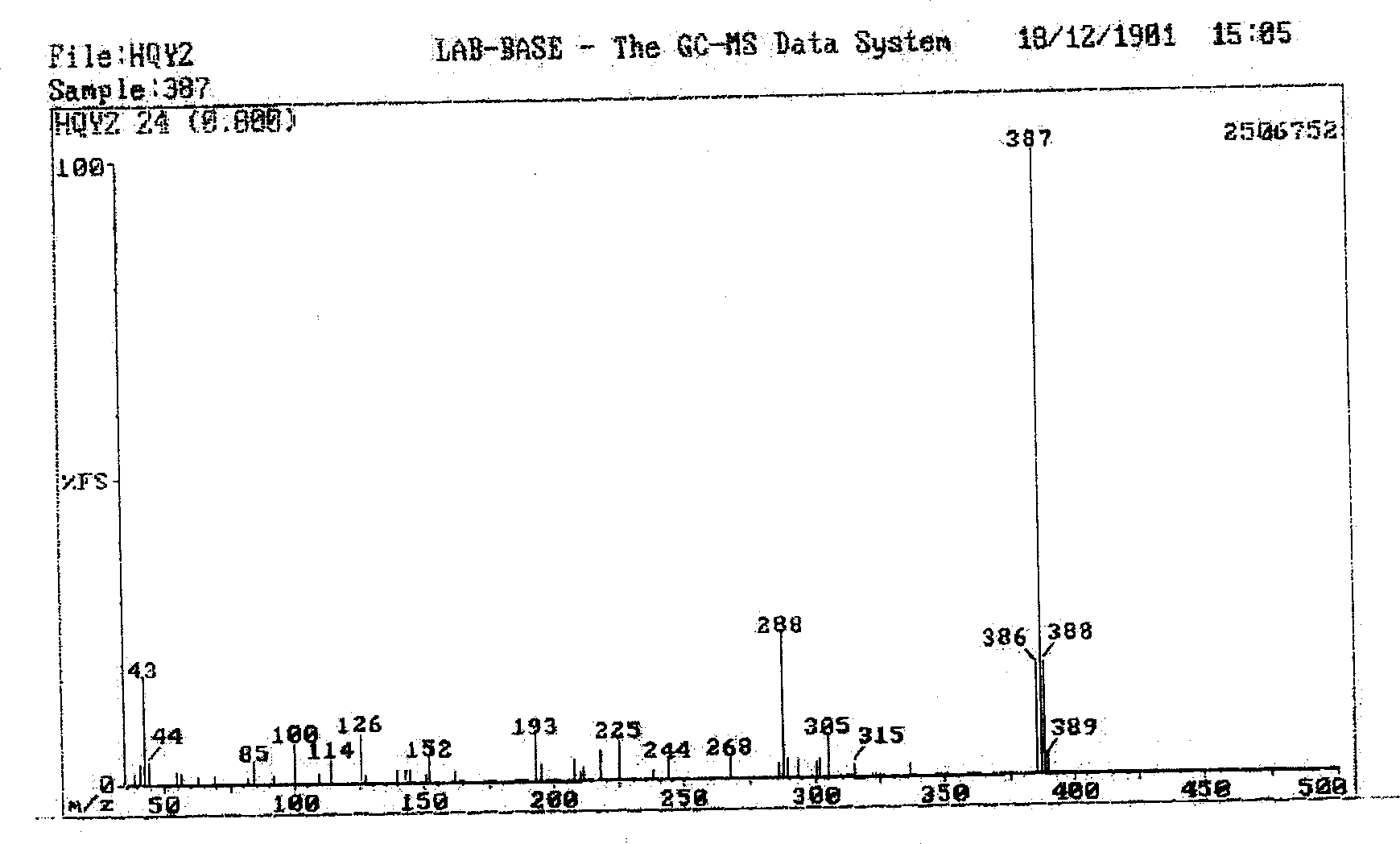
[0054] Synthesized according to the method of compound 1, replacing aluminum chloride with gallium chloride, the pure product of (acetylacetonate)-(salicylaldehyde orthoaniline phenol)gallium(III) (compound 2) can be obtained. Mass spectrum: m / e, 380; elemental analysis (C18H16GaNO4): experimentally determined C: 58.007%, H: 4.309% N: 3.759%; theoretical value: C: 58.065%, H: 4.301%, N: 3.763%
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com