Iron-based catalyst prepared by coprecipitation-melting method, preparation method and application thereof
A technology of iron-based catalyst and co-precipitant, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. Industrial production, reduction of mechanical strength and other problems, to achieve the effects of inhibiting carbon deposition, improving utilization, and improving lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
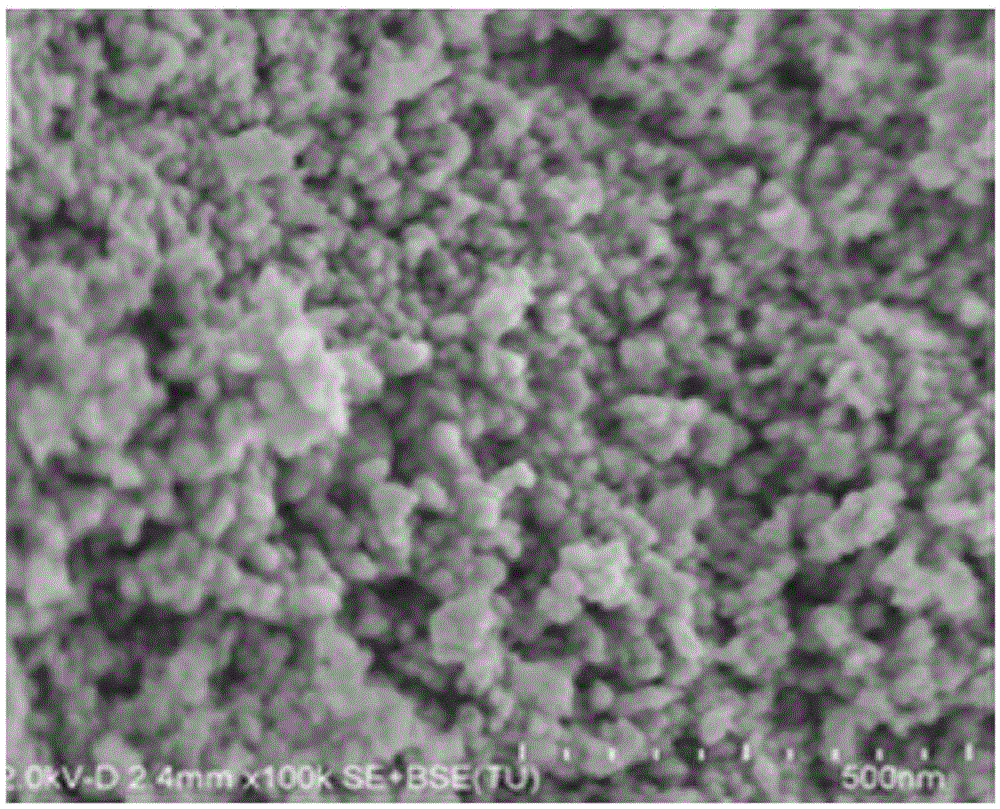
[0040] The preparation of embodiment 1 catalyst sample
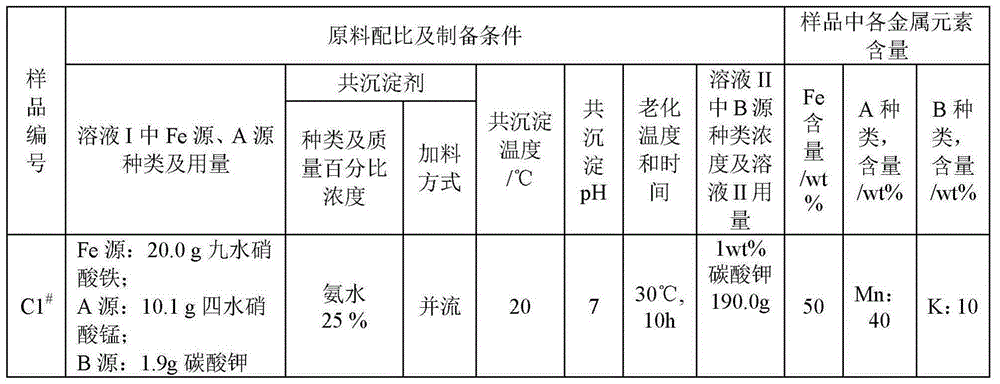
[0041] Dissolve 20.0g ferric nitrate nonahydrate and 10.1g manganese nitrate hexahydrate in 200ml deionized water to obtain solution I. The co-precipitant is ammonia water with a concentration of 25% by mass percentage, and continuously mix the solution I and the coprecipitant in parallel flow mode , control the mixing ratio to keep the pH of the solution at 7, keep the temperature at 20°C during the co-precipitation process, and continuously stir during the precipitation process. After the co-precipitation is completed, age at a temperature of 30° C. for 10 h, filter, wash with deionized water until neutral, and add 190 g of 1 wt % potassium carbonate solution (solution II) to the filter cake for repulping to obtain mixture I. The mixture I was dried at a temperature of 50°C for 12h, and then fired at a temperature of 500°C in an air atmosphere for 10h. Then melt treatment at a temperature of 1200°C for 10 hours in an ...
Embodiment 3
[0059] Example 3 Sample C1 # ~Sample C15 # and sample D1 # , Sample D2 # Reaction Performance Test of Syngas to Light Olefins
[0060] 2g sample C1 # Put it into the reaction tube, and combine the liner, quartz wool and quartz sand to keep the catalyst in the constant temperature section. The reaction tube was installed in a fixed bed apparatus so that the thermocouple was located on the catalyst bed. Use H 2 Carry out reduction treatment, the reduction conditions are as follows: the reduction temperature is 350° C., the reduction is at normal pressure, and the reduction time is 6 hours. The reduced catalyst is used for the reaction of synthesis gas to produce light olefins, the reaction conditions are 350°C, the reaction pressure is 4MPa, the volume ratio of raw gas is H 2 / CO=0.5, airspeed GHSV=1000h -1 . At the same time, set the valve box temperature, pipeline insulation and chromatographic pipeline insulation to 150°C. The online chromatographic detection of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com