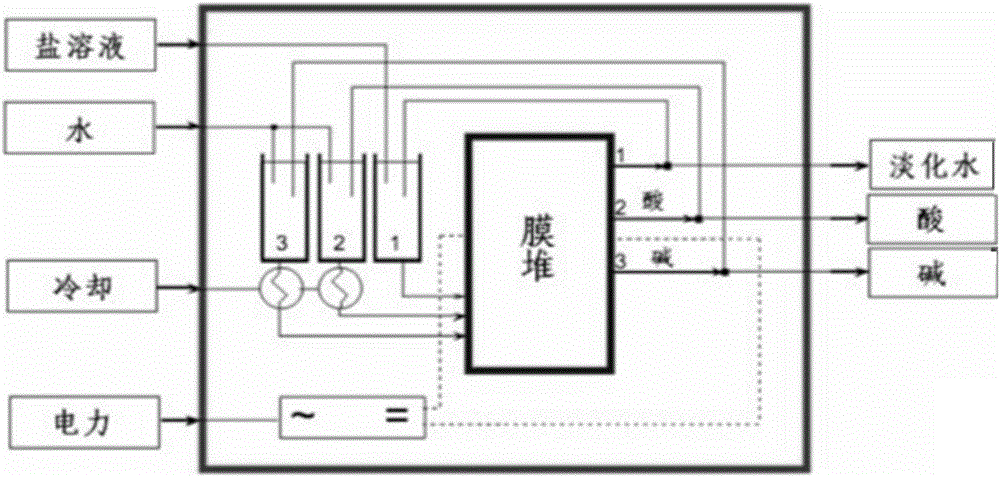
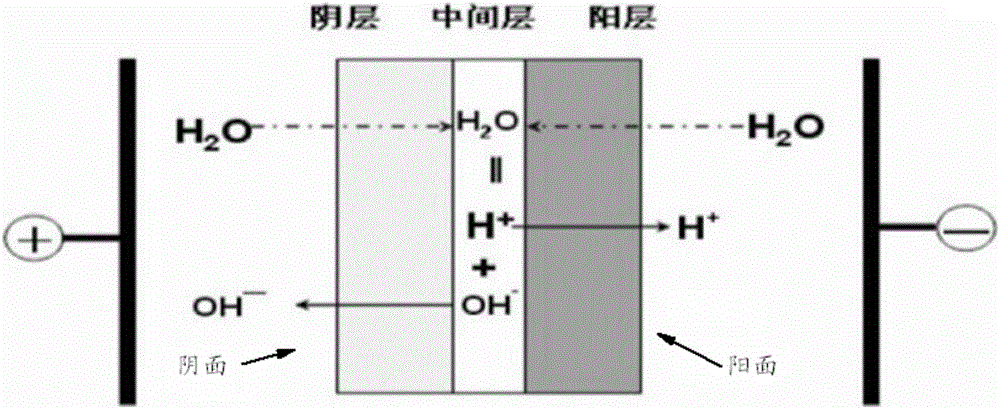
Methods for preparing halogen ethanol and ethylene oxide
A technology of ethylene oxide and haloethanol, which is applied in the fields of preparing ethylene oxide by using a new halohydrin method, preparing haloethanol, and preparing ethylene oxide, can solve the problem of large consumption of water resources, high COD of waste water, and dangerous production. big problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] 1) Catalyst acid treatment: soak the TS-1 molecular sieve in hydrochloric acid with a mass fraction of 36% for 1 hour, then wash the catalyst with distilled water, and dry the catalyst for 6 hours at 40°C;
[0112] 2) Haloalcoholization: Add 70wt% concentration of hydrogen peroxide H 2 o 2 , HCl solution (hydrochloric acid) and ethene of 35wt% concentration, carry out chloroalcoholization reaction at the temperature of 45 ℃, wherein the hydrogen peroxide H of 70wt% concentration 2 o 2 , 35wt% concentration of HCl solution and ethylene flow should make H 2 o 2 The molar ratio of HCl to ethylene is about 1.2:1.2:1. This gives the halohydrin, a mixture of 2-chloroethan-1-ol and 1-chloroethan-2-ol.
Embodiment 2
[0117] 1) Catalyst acid treatment: soak TS-1 molecular sieve in hydrochloric acid with a mass fraction of 25% for 2 hours, then wash the catalyst with distilled water, and dry the catalyst for 2 hours at 40°C; among them, the synthesis of TS-1 The method refers to Example 1 of CN201410812216.8.
[0118] 2) Haloalcoholization: add TS-1 molecular sieve, 35wt% concentration of hydrogen peroxide H in the tower reactor 2 o 2 , HCl solution (hydrochloric acid) and ethylene of 20wt% concentration, carry out chlorohydrinization reaction at the temperature of 35 ℃, wherein the mass ratio of TS-1 molecular sieve and ethylene is 0.05:1, the hydrogen peroxide H of 35wt% concentration 2 o 2 , 20wt% concentration of HCl solution and ethylene should be added in such a way that H 2 o 2 The molar ratio of HCl to ethylene is about 1.5:1.1:1. This gives the halohydrin, a mixture of 2-chloroethan-1-ol and 1-chloroethan-2-ol.
Embodiment 3
[0130] 1) Catalyst acid treatment: Soak TS-1 molecular sieve in hydrochloric acid with a mass fraction of 36% for 1 hour, then wash the catalyst with distilled water, and dry the catalyst for 6 hours at 40°C; among them, the synthesis of TS-1 The method refers to Example 1 of CN201410812216.8.
[0131] 2) Haloalcoholization: Add 70wt% concentration of hydrogen peroxide H 2 o 2 , HCl solution (hydrochloric acid) and ethene of 35wt% concentration, carry out chloroalcoholization reaction at the temperature of 45 ℃, wherein the hydrogen peroxide H of 70wt% concentration 2 o 2 , 35wt% concentration of HCl solution and ethylene flow should make H 2 o 2 The molar ratio of HCl to ethylene is about 1.2:1.2:1. This gives the halohydrin, a mixture of 2-chloroethan-1-ol and 1-chloroethan-2-ol.
[0132] 3) Saponification: saponifying the haloalcohol obtained in step 2) with sodium hydroxide, and separating to obtain an ethylene oxide organic phase and a sodium chloride solution. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com