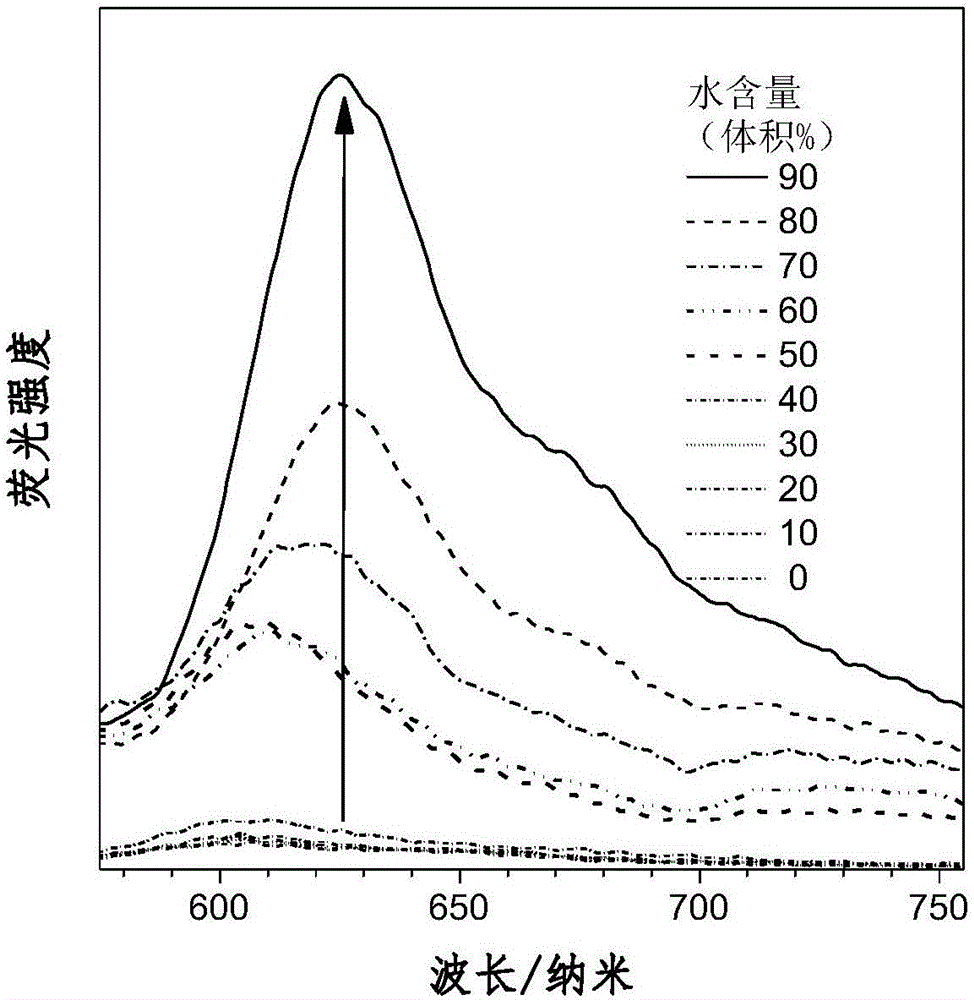
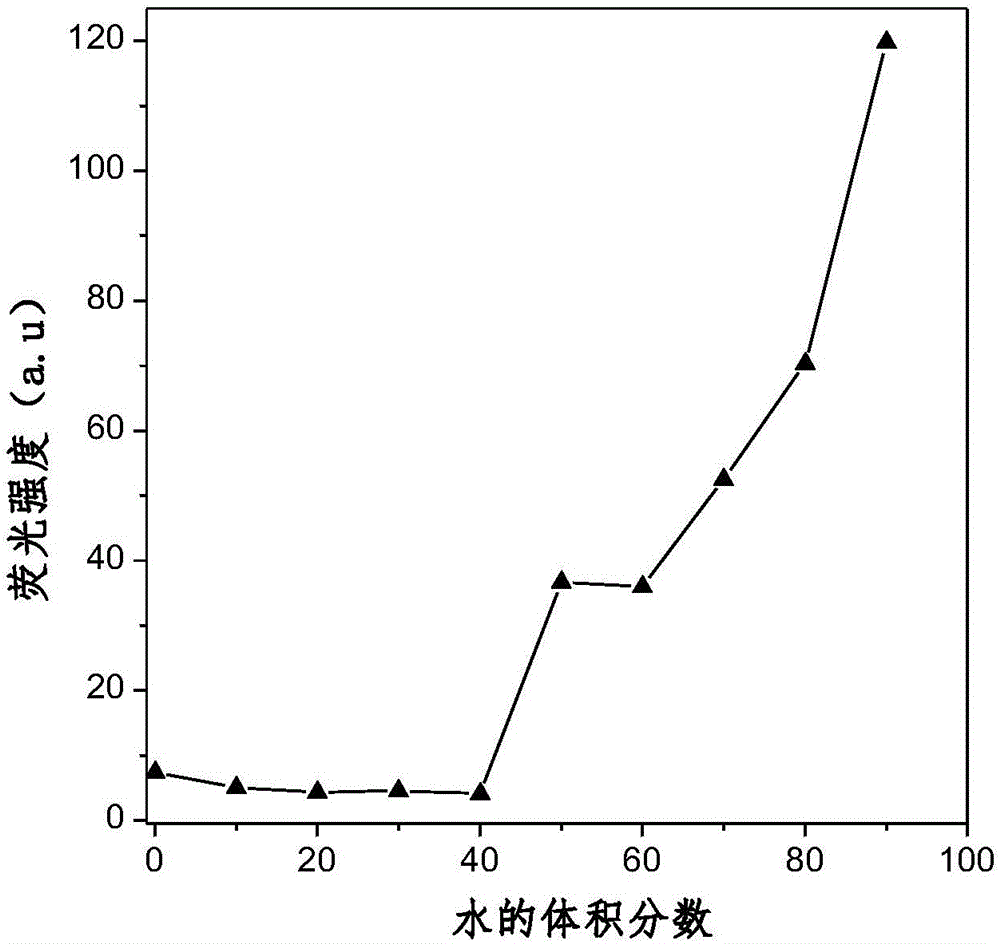
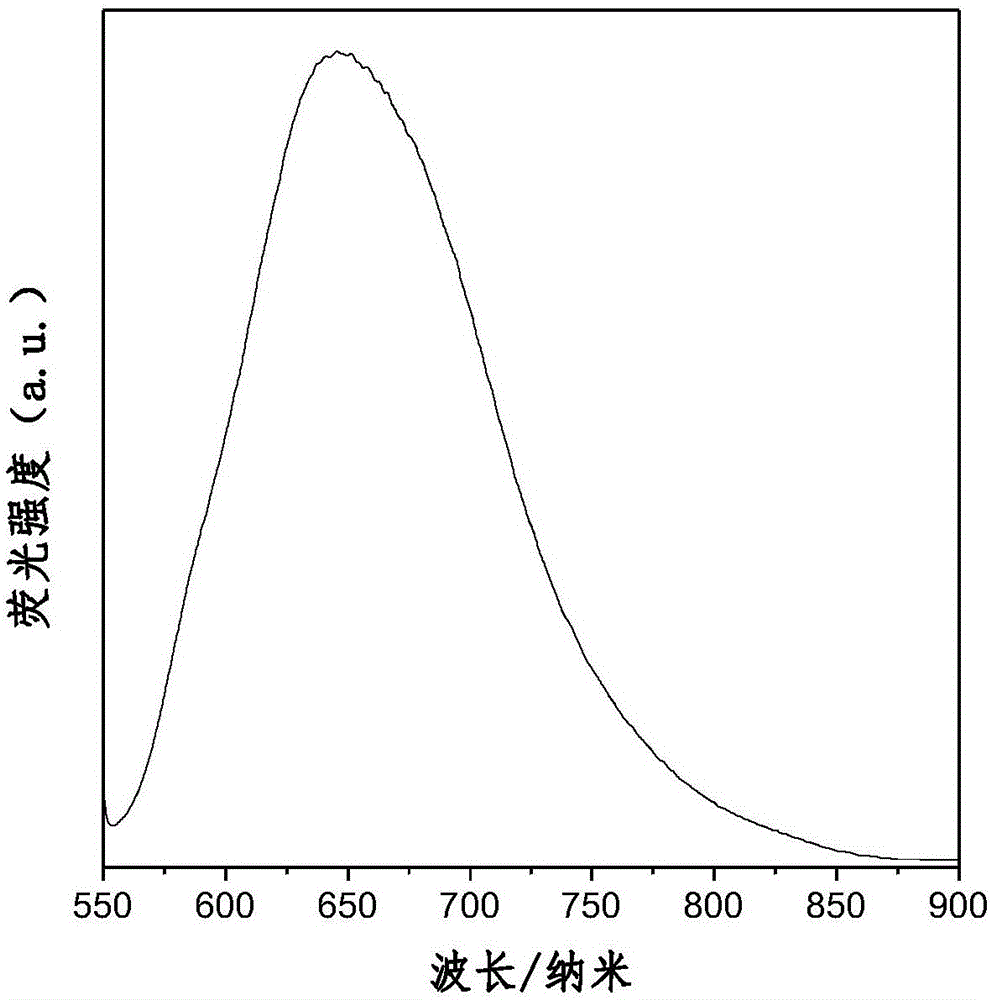
Aggregation-induced red light emission material and preparation method thereof
An aggregation-inducing and red-emitting technology, which is applied in the fields of luminescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of harsh reaction conditions, aggregation and quenching of organic red light materials, and high cost, and achieve mild reaction conditions and experimental The effect of simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] In a preferred technical solution of the present invention, R 1 is n-butyl, R 2 for -H.
[0054] (1) Synthesis of intermediate 1-(4-hydroxyphenyl)-1,2,2-triphenylethylene. 4-Hydroxybenzophenone (1.9 g, 10 mmol), benzophenone (2.2 g, 12 mmol), and Zn powder (2.9 g, 44 mmol) were added to a 250 mL round bottom flask. After vacuuming and changing nitrogen three times, 80 mL of tetrahydrofuran was added. After cooling to 0°C, slowly add TiCl 4 (4.2 g, 22 mmol), followed by stirring for 1 h. Then stir at 70°C for 24h, after cooling to room temperature, add 80mL of dilute hydrochloric acid (1mol / L) to adjust to neutrality, extract three times with DCM, collect the organic layer, dry over anhydrous magnesium sulfate, and spin the solvent to obtain the crude product. A 20:1 mixture of petroleum ether and ethyl acetate as eluent, SiO 2 As a stationary phase, 0.8 g of a white solid was obtained by column chromatography, and the yield was 47%.
[0055]
[0056] (2) Synth...
Embodiment 2
[0064] In another preferred technical solution of the present invention, R 1 is cyclohexyl, R 2 for -CH 3 .
[0065] (1) Synthesis of intermediate 4-(1,2-diphenyl-2-p-tolylvinyl)phenol. 4-Hydroxybenzophenone (1.9 g, 10 mmol), 4-methylbenzophenone (2.9 g, 15 mmol), and Zn powder (3.9 g, 60 mmol) were added to a 250 mL round bottom flask. After vacuuming and changing nitrogen three times, 80 mL of tetrahydrofuran was added. After cooling to 0°C, slowly add TiCl 4 (5.7g, 30mmol), followed by stirring for 0.8h. Then stir at 65°C for 36h, after cooling to room temperature, add 80mL of dilute hydrochloric acid (1mol / L) to adjust to neutrality, extract three times with DCM, collect the organic layer, dry over anhydrous magnesium sulfate, and spin the solvent to obtain the crude product. A 40:1 mixture of petroleum ether and ethyl acetate as eluent, SiO 2 As a stationary phase, 0.76 g of a white solid was separated by column chromatography, and the yield was 42%.
[0066]
...
Embodiment 3
[0073] In another preferred technical solution of the present invention, R 1 is 2-ethylhexyl, R 2 for-OCH 3 .
[0074] (1) Synthesis of intermediate 4-(2-(4-methoxyphenyl)-1,2-diphenylvinyl)phenol. Into a 250 mL round bottom flask were added 4-hydroxybenzophenone (1.9 g, 10 mmol), 4-methoxybenzophenone (2.1 g, 10 mmol), Zn powder (2.6 g, 40 mmol). After vacuuming and changing nitrogen three times, 80 mL of tetrahydrofuran was added. After cooling to 0°C, slowly add TiCl 4 (3.8g, 20mmol), then stirred for 1h, then stirred at 85°C for 12h, after cooling to room temperature, added 80mL of dilute hydrochloric acid (1mol / L) to adjust to neutral, extracted three times with DCM, collected the organic layer, anhydrous magnesium sulfate Drying, spin-drying solvent obtains crude product, is the eluent with the mixture of sherwood oil and ethyl acetate of 60:1 with the volume ratio, SiO 2 As a stationary phase, 0.87 g of a white solid was obtained by column chromatography, with a y...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum emission wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com