Isoflavone derivative, preparation method and application thereof
A technology for compounds and solvates, applied in the field of isoflavone derivatives and their preparation, achieves the effects of broad market prospect and simple and efficient preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
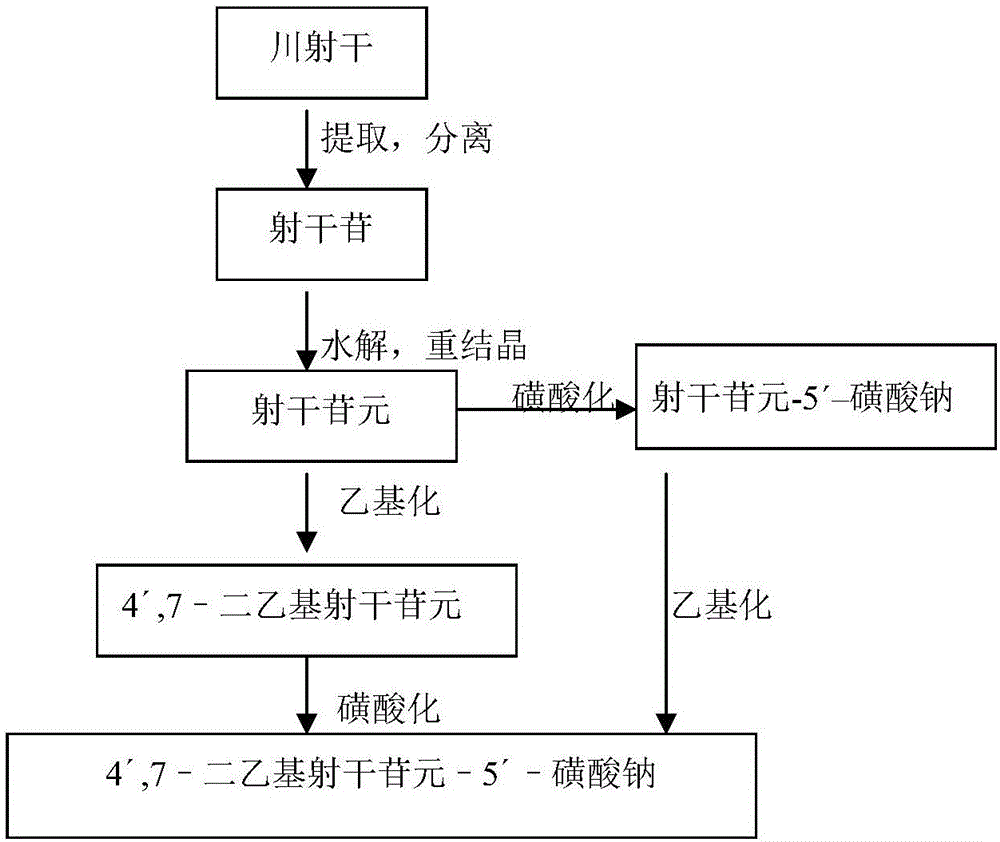
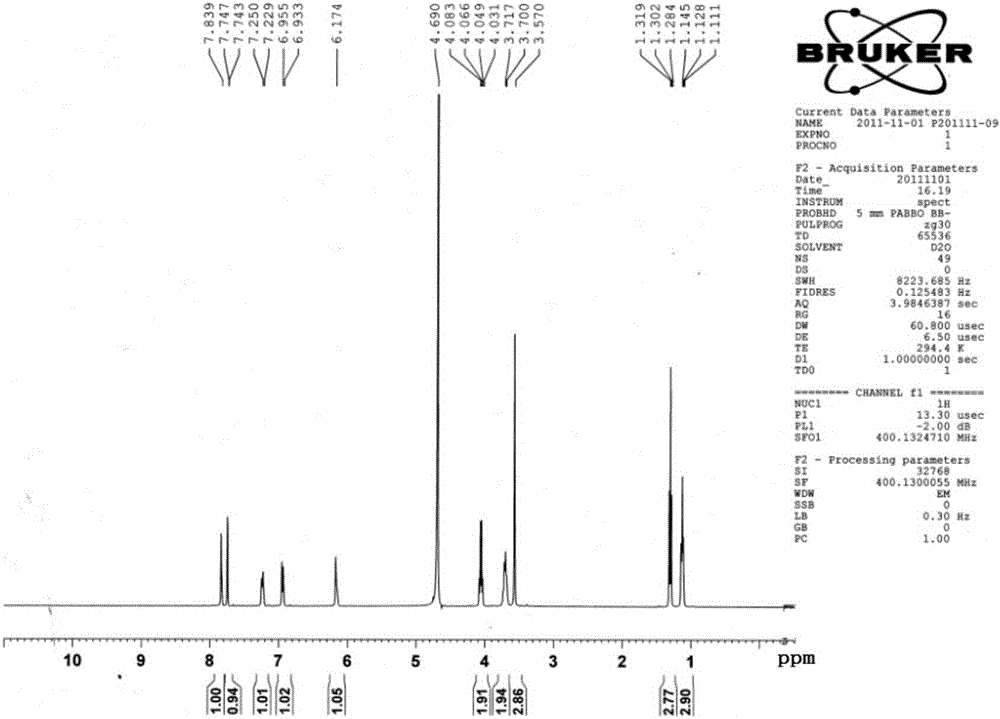
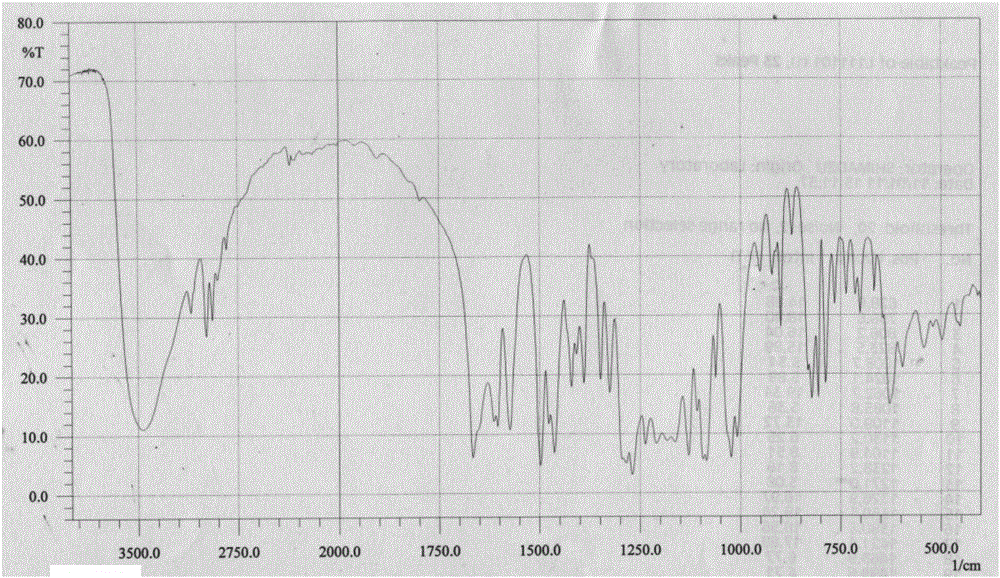
[0031] Example 1 Preparation of the compound 4',7-diethylaglycone-5'-sulfonate (MJJ-S) of the present invention
[0032]
[0033] 1. Extraction and separation of Sheganoside
[0034] Chuanshegan crude drug was dried at 60°C, crushed into coarse powder and passed through a 20-mesh sieve, placed in an extraction container, heated and refluxed with 95% ethanol to extract 3 times, each time 1 hour, and the amount of each solvent was 4 times that of the medicinal material ( v / w). Filtrate while hot, combine filtrate, 60 DEG C depressurize recovery solvent, obtain the syrup-like substance (brown yellow extract) of specific gravity 1.2g / mL (60 DEG C measure), its yield is 49~51% (w / w). Treat the above-mentioned extract with chloroform, recover the solvent, add 95% ethanol (1 / 5 of the medicinal material, v / w) to the residue, stir evenly, and filter with suction to obtain the crude shegan glycoside. Take the crude product of Sheganoside, add 95% ethanol and heat it under reflux ...
Embodiment 2
[0044] Example 2 Preparation of the compound 4′,7-diethylaglycone-5′-sulfonate (MJJ-S) of the present invention
[0045] ① Shegan aglycon + diethyl carbonate → 4′,7-diethylshegan aglycone
[0046]
[0047]Take 100g of aglycone, add 20g of NaOH, mix well, add 300mL of 95% ethanol to a 2-liter round bottom flask, heat and boil in a water bath for 5 minutes, add 200mL of diethyl carbonate, react for half an hour, take it out, and use immediately Adjust the pH to 2-5 with hydrochloric acid, add water, stir and let cool. Filter to obtain a colorless powder, stir evenly with 95% ethanol, filter, wash repeatedly until the filtrate is nearly colorless, dry under reduced pressure at 60°C to obtain 100g of 4′,7-diethylsheganaglycone (light yellow crystalline powder, yield 70%). The sulfonation reaction is the same as in Example 1.
Embodiment 3
[0048] Example 3 Preparation of the compound 4′,7-diethylaglycone-5′-sulfonate (MJJ-S) of the present invention
[0049]
[0050]
[0051] Take Shegan aglycone 100g, iodoethane 100g, K 2 CO 3 100g was dissolved in 500mL DMF, and reacted for 6 hours at 60°C under the action of 40HZ ultrasonic waves. After the reaction was completed, the reactant was cooled to room temperature, and the insoluble matter was filtered off. The filtrate was distilled under reduced pressure to obtain a light yellow solid, which was recrystallized in chloroform to obtain 4′ , 7-diethyl shegan aglycone 100g (light yellow crystalline powder, yield 70%).
[0052] The sulfonation reaction is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com