Polycyclic aromatic hydrocarbon derivative containing naphthyridine group and application of polycyclic aromatic hydrocarbon derivative
A technology containing naphthyridine groups and fused-ring aromatics, which is applied in electrical components, circuits, organic chemistry, etc., can solve the problems of increasing the complexity and disadvantages of the device manufacturing process, reducing the cost of OLEDs, etc., achieving good electron acceptance and reducing The effect of operating voltage and improving luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1 The synthesis of various 3-bromo-7-aryl-1,5-naphthyridine derivative intermediates
[0065] Synthesis of 1,3-bromo-7-phenyl-1,5-naphthyridine
[0066]
[0067] 1000 ml three-necked flask equipped with magnetic stirring and nitrogen protection, add 5.72 g of 3,7-dibromo-1,5-naphthyridine (molecular weight 286, 0.02 mol), 2.7 g of phenylboronic acid (molecular weight 122, 0.022 mol), four ( (Triphenylphosphine) palladium 1.16g (molecular weight 1154, 0.001mol), 2M sodium carbonate aqueous solution 80ml, toluene 80ml, ethanol 80ml. After argon displacement, reflux at 80 ℃, use thin layer chromatography (TLC) method Monitor the reaction, after 4 hours TLC found that the raw material dibromide had reacted completely, only the monobromide product point. Cool to 25°C, separate the organic layer, evaporate to dryness, separate by column chromatography, wash with ethyl acetate / petroleum ether, obtain 5.77 g Target intermediate 3-bromo-7-phenyl-1,5-naphthyridine,...
Embodiment 2
[0075] Synthesis of compound shown in formula (14)
[0076]
[0077] 1000 ml bottle with magnetic stirring, add 5.68 g of 3-bromo-7-phenyl-1,5-naphthyridine (molecular weight 284, 0.02 mol), 9,10-di(naphthalene-2-yl)anthracene-2 - boric acid 11.0g (molecular weight 474, 0.022mol), tetrakis ((triphenylphosphine) palladium 1.16g (molecular weight 1154, 0.001mol), 2M aqueous sodium carbonate solution 80ml, toluene 80ml, ethanol 80ml. After argon replacement, Reflux at 80 DEG C, monitor the reaction with thin-layer chromatography (TLC), after 3.5 hours, TLC finds that the raw material bromide has reacted completely, and only the product point. Cool down, separate the organic layer, evaporate to dryness, separate by column chromatography, ethyl acetate / Petroleum ether was rinsed to obtain 11.33 g of the compound represented by formula (14), with a molecular weight of 634 and a yield of 89.3%.
[0078] Product MS (m / e): 634, elemental analysis (C 48 h 30 N 2 ): theoretical v...
Embodiment 3
[0080] Synthesis of compound shown in formula (15)
[0081] The synthesis steps are the same as in Example 2, except that 3-bromo-7-phenyl-1,5-naphthyridine is changed to 3-bromo-7-(naphthalene-1-yl)-1,5-naphthyridine, other reagents Without changing, the compound represented by formula (15) is obtained.
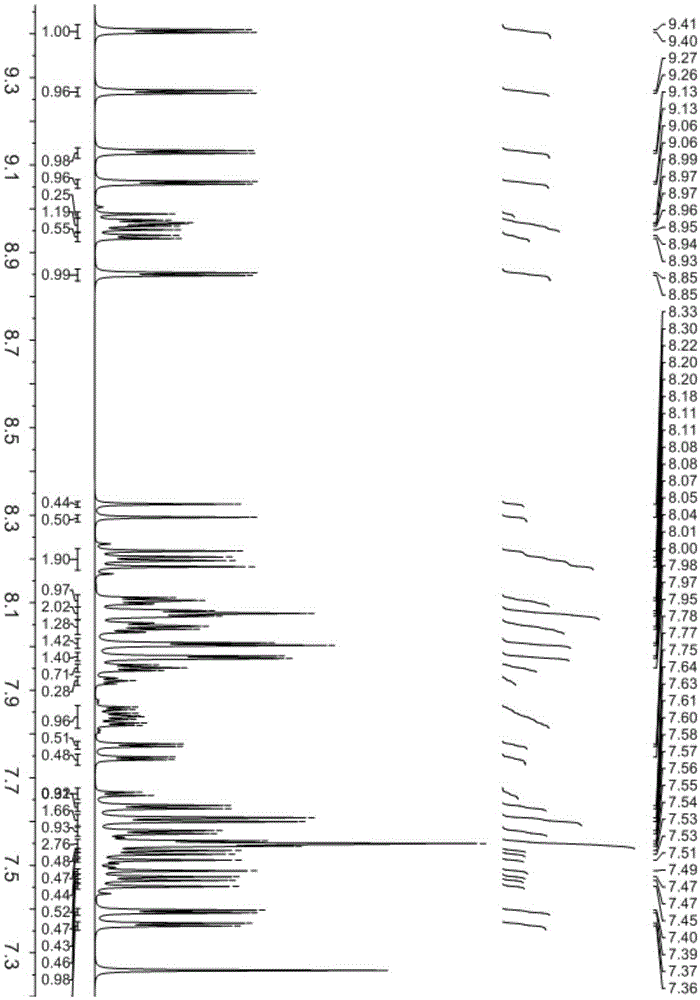
[0082] Product MS (m / e): 684, elemental analysis (C 52 h 32 N 2 ): theoretical value C: 91.20%, H: 4.71%, N: 4.09%; measured value C: 91.23%, H: 4.73%, N: 4.04%, its nuclear magnetic spectrum ( 1 HNMR) see figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com