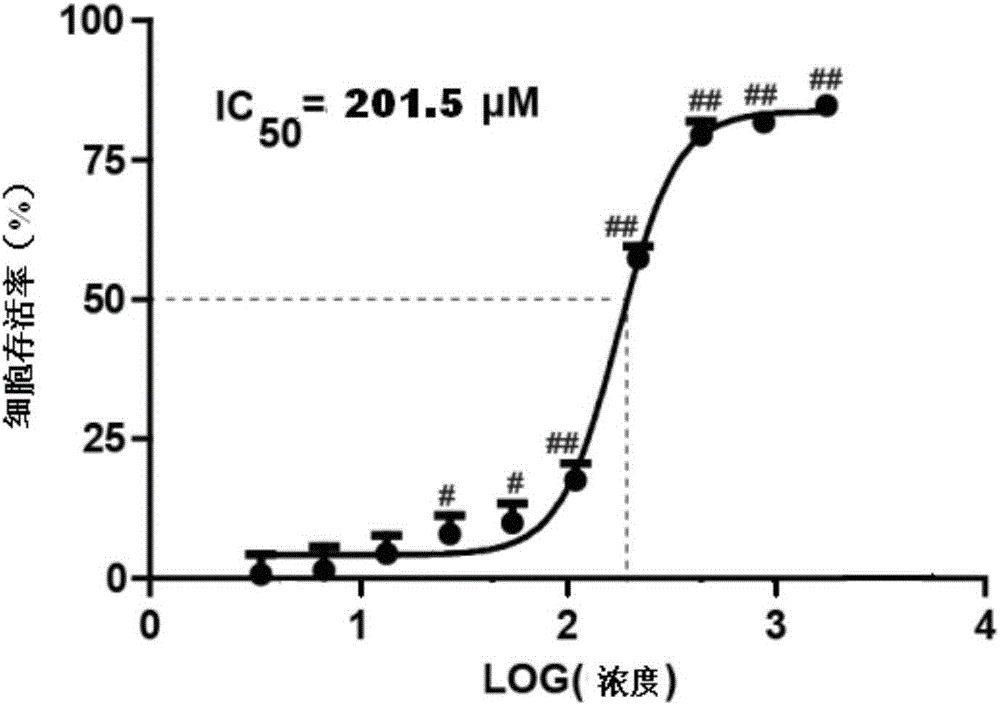
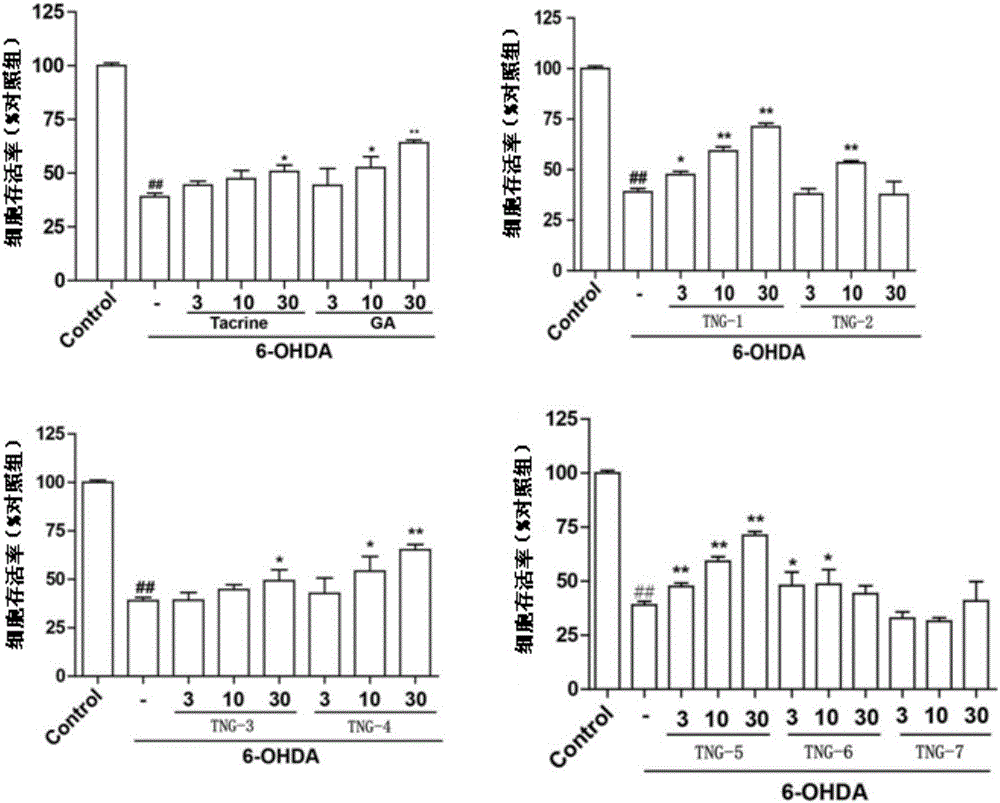
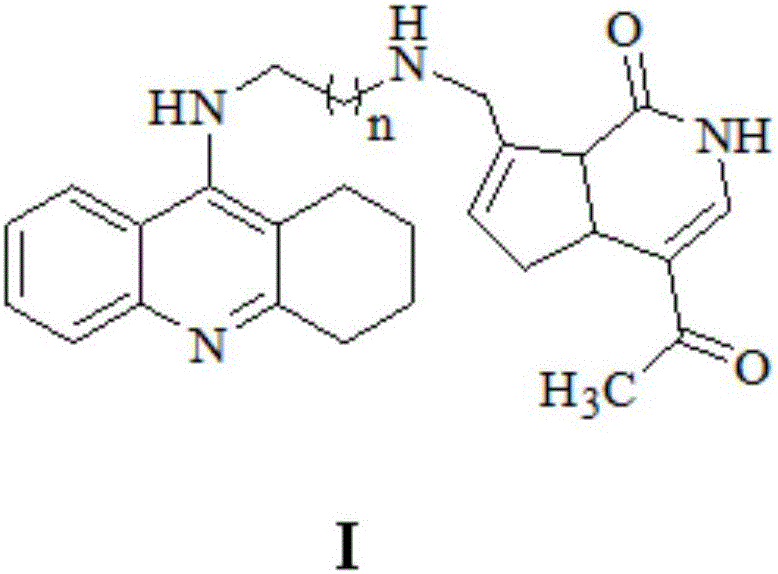
Gardenamide A-tacrine diad compound as well as preparation method and application thereof
A technology of gardenia amide and compound, applied in the field of medicinal chemistry, can solve problems such as need for in-depth research, lack of therapeutic drugs, etc., and achieve the effect of delaying degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1: Synthesis of 10-N-(2-(10-aminogardeninamide A)amino)ethyltacrine (TNG-1)
[0063] Include the following steps:
[0064] (1) Preparation of intermediate compound G-2: Weigh genipin (340.2mg, 1.5mmol) and imidazole (308.5mg, 4.5mmol) in a 25mL round bottom flask, dissolve with 3mL dry tetrahydrofuran solution; then Dimethyl tert-butyl silicon chloride (TBSCl) (408.8 mg, 2.7 mmol) was weighed, dissolved in dry tetrahydrofuran solution, and added dropwise to the reaction system. The reaction was stirred at room temperature for 1.5 hours. After the reaction was completed, the solvent was directly spin-dried. The residue was separated and purified by liquid column chromatography (petroleum ether: ethyl acetate = 10:1 (V:V)) to obtain 451.2 mg of a white compound with a yield of 89%. The characterization data for the white compound is 1 H NMR (300MHz, CDCl 3 )δ: 7.55 (s, 1H, 3-H), 5.83 (brs, 1H, 1-H), 4.80 (d, J = 12Hz, 1H, CH), 4.31-4.25 (q, J = 12, 18Hz, 2H)...
Embodiment 2
[0074] Example 2: Synthesis of 10-N-(3-(10-aminogardeninamide A) amino) n-propyl tacrine (TNG-2)
[0075] Include the following steps:
[0076] (1) Preparation of intermediate compound DG: prepared according to steps (1) to (4) of Example 1;
[0077] (2) Preparation of intermediate compound T-4: prepared according to steps (5) to (7) of Example 1;
[0078] (3) Preparation of intermediate compound TN-2: prepared according to step (8) of Example 1. Ethylenediamine (8.65mmol) was replaced by 1,3-propanediamine (8.65mmol). 0.25g of yellow oily liquid was obtained, and the yield was about 85%. 1 H NMR (300MHz, CDCl 3 )δ: 8.16-8.13(d, J=9Hz, 1H), 7.88-7.85(d, J=9Hz, 1H), 7.64-7.59(m, 1H), 7.44-7.38(m, 1H), 4.1(s ,2H,-NH 2 ), 2.66(t, J=12Hz, 2H), 2.39(t, J=12Hz, 2H), 2.27-2.19(m, 2H), 1.55-1.42(m, 4H). The above data confirm that this compound is an intermediate compound TN-2;
[0079] (4) Preparation of 10-N-(3-(10-aminogardeninamide A)amino)n-propyltacrine (TNG-2): prepared ...
Embodiment 3
[0081] Example 3: Synthesis of 10-N-(4-(10-aminogardeninamide A) amino) n-butyltacrine (TNG-3)
[0082] Include the following steps:
[0083] (1) Preparation of intermediate compound DG: prepared according to steps (1) to (4) of Example 1;
[0084] (2) Preparation of intermediate compound T-4: prepared according to steps (5) to (7) of Example 1;
[0085] (3) Preparation of intermediate compound TN-3: prepared according to step (8) of Example 1. Ethylenediamine (8.65mmol) was replaced by 1,4-butanediamine (8.65mmol). 0.25 g of yellow oily liquid was obtained, and the yield was about 81%. 1 H NMR (300MHz, CDCl 3 )δ:7.94-7.92(d,J=6.0Hz,1H),7.88-7.86(d,J=6.0Hz,1H),7.53-7.49(m,1H),7.32-7.29(m,1H),4.18 (s,1H,-NH-),3.47(t,J=12.0Hz,2H),3.01(m,2H),2.94(m,4H),2.71-2.66(m,4H),1.67-.62( m,2H), 1.54-1.49(m,2H). The above data confirm that this compound is the intermediate compound TN-3.
[0086] (4) Preparation of 10-N-(4-(10-aminogardeninamide A)amino)n-butyltacrine (TNG-3): prepa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com