Branching macromolecule with cyclotriphosphazene as structural framework and photoresist composition prepared through branching macromolecule
A technology of structural skeleton and cyclotriphosphazene, which is applied in the field of branched macromolecules synthesized based on cyclotriphosphazene to achieve the effects of good product performance, mild reaction conditions and high resolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
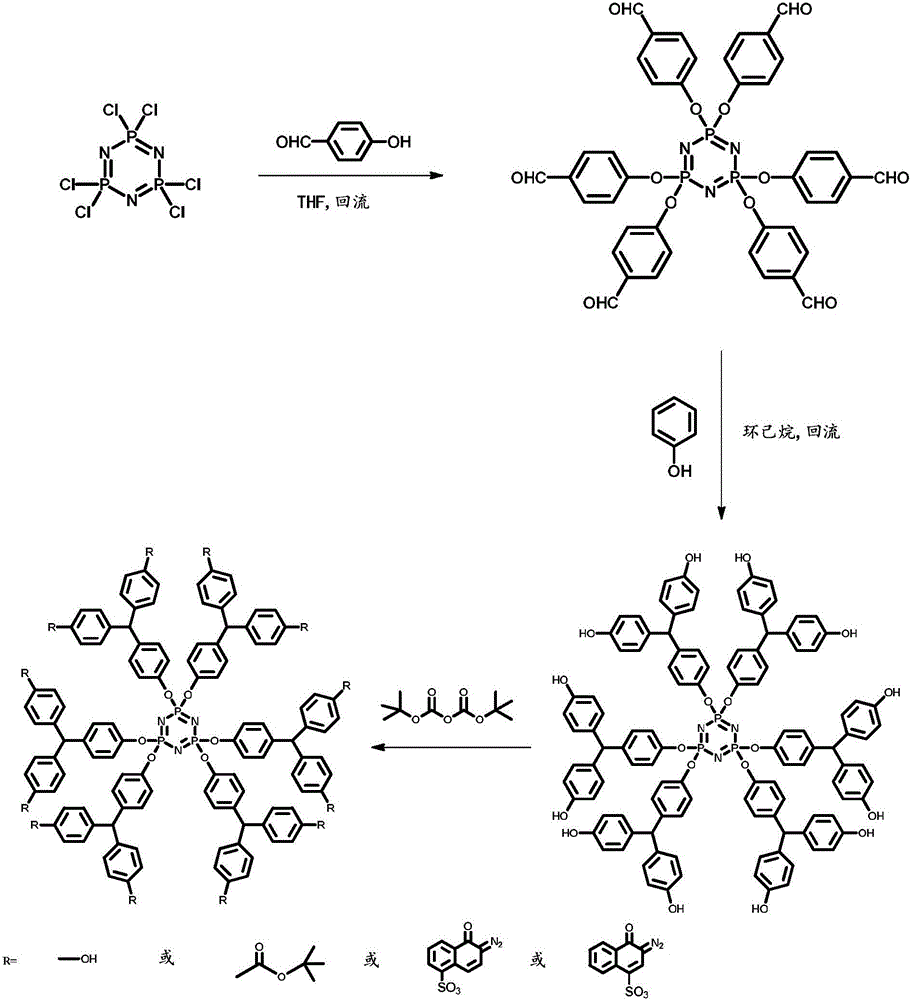
[0030] refer to figure 1 , a kind of branched macromolecule taking cyclotriphosphazene as structural skeleton, the preparation method of described branched macromolecule is:
[0031] (1) Take p-hydroxybenzaldehyde (14.76g, 0.12mmol) in a 250mL single-mouth bottle, add 50mL tetrahydrofuran and 20mL triethylamine, put the single-mouth bottle in an oil bath, heat up to 65°C, and take hexachlorocyclotri Phosphazene (6.95g, 0.02mmol) was dissolved in 40mL of tetrahydrofuran, added dropwise within 40min, stirred and refluxed for 24h, and the resulting white suspension was suction filtered to obtain a yellow filtrate, which was removed by rotary evaporation, washed twice with ethanol, and pumped White powder was obtained after filtration, recrystallized twice from ethyl acetate, and suction filtered to obtain white powder, which was hexa-formylphenoxycyclotriphosphazene;
[0032] (2) In a 250mL three-necked flask equipped with a magnetic stirrer, a thermometer, and a water separator...
Embodiment 2
[0046] refer to figure 1 , a kind of branched macromolecule taking cyclotriphosphazene as structural skeleton, the preparation method of described branched macromolecule is:
[0047] (1) Take p-hydroxybenzaldehyde (7.38g, 0.06mmol) in a 250mL one-mouth bottle, add 50mL tetrahydrofuran and 20mL triethylamine, put the one-mouth bottle in an oil bath, heat up to 65°C, and take hexachlorocyclotri Phosphazene (6.95g, 0.02mmol) was dissolved in 40mL of tetrahydrofuran, added dropwise within 40min, stirred and refluxed for 24h, and the resulting white suspension was suction filtered to obtain a yellow filtrate, which was removed by rotary evaporation, washed twice with ethanol, and pumped White powder was obtained after filtration, recrystallized twice from ethyl acetate, and suction filtered to obtain white powder, which was hexa-formylphenoxycyclotriphosphazene;
[0048] (2) In a 250mL three-necked flask equipped with a magnetic stirrer, a thermometer, and a water separator, add h...
Embodiment 3
[0053] refer to figure 1 , a kind of branched macromolecule taking cyclotriphosphazene as structural skeleton, the preparation method of described branched macromolecule is:
[0054] (1) Take p-hydroxybenzaldehyde (14.76g, 0.12mmol) in a 250mL single-mouth bottle, add 50mL tetrahydrofuran and 20mL triethylamine, put the single-mouth bottle in an oil bath, heat up to 65°C, and take hexachlorocyclotri Phosphazene (6.95g, 0.02mmol) was dissolved in 40mL of tetrahydrofuran, added dropwise within 40min, stirred and refluxed for 24h, and the resulting white suspension was suction filtered to obtain a yellow filtrate, which was removed by rotary evaporation, washed twice with ethanol, and pumped White powder was obtained after filtration, recrystallized twice from ethyl acetate, and suction filtered to obtain white powder, which was hexa-formylphenoxycyclotriphosphazene;
[0055] (2) In a 250mL three-necked flask equipped with a magnetic stirrer, a thermometer, and a water separator...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com