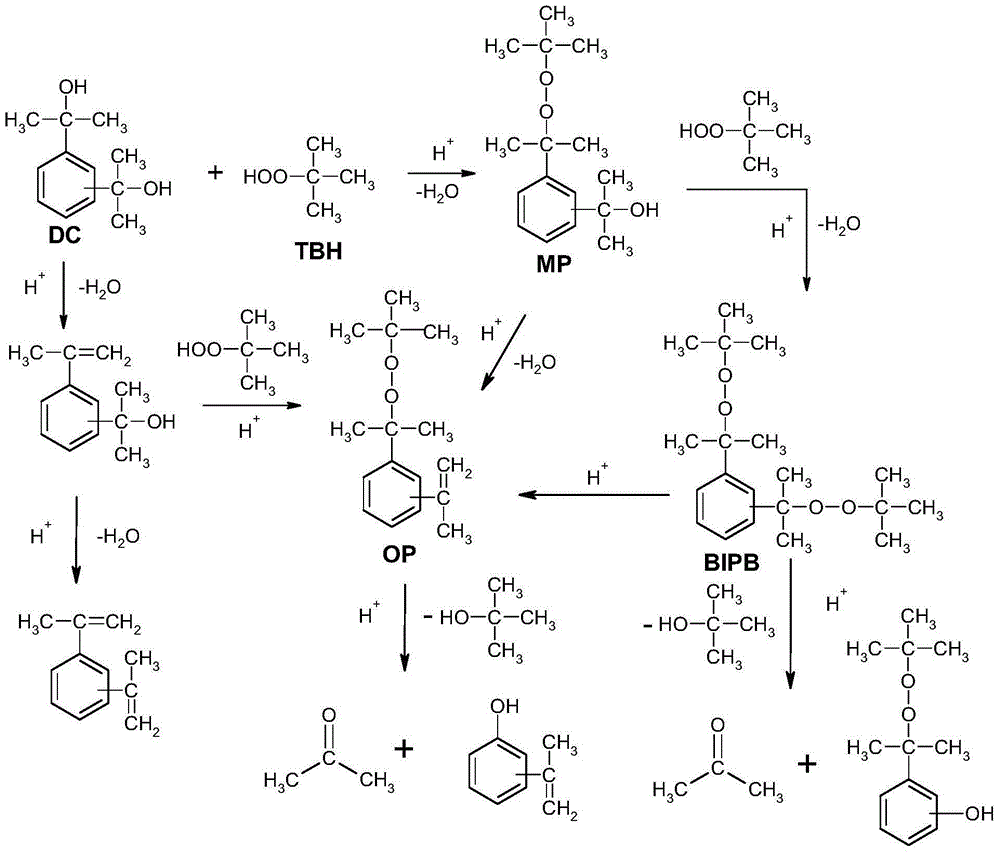
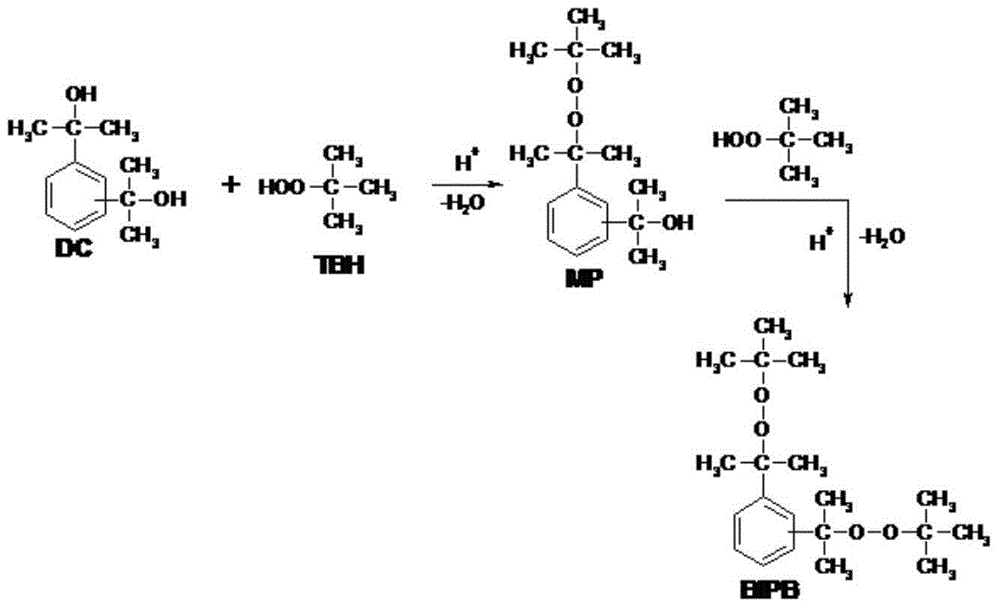
Bis(t-butylperoxyisopropyl)benzene production method
A technology of isopropyl tert-butyl peroxide and isopropyl tert-butyl peroxide is applied in the production field of organic peroxides, which can solve the problems of unfavorable environmental protection, instability of strong acid and high temperature, and high cost of raw materials, and achieve The effects of eliminating the DC drying process, increasing the yield and content, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] (1) Put 1200 kilograms of toluene into the condensation reaction kettle and start stirring. Put 624 kilograms of 70wt% TBHP aqueous solution into the condensation tank, control the temperature at 25° C., stir for 20 minutes, settle statically for 20 minutes, and remove the lower aqueous phase (about 160 kg).
[0070](2) Put 400 kg of refined DC (97.5%), 3.2 kg of 50wt% perchloric acid aqueous solution and 3.2 kg of tap water mixture into the condensation reactor at a temperature of about 20°C, wherein DC is a mixture of m- and para-isomers , the ratio of the meta-isomer of bis-(2-hydroxyisopropyl) benzene to the para-isomer of bis-(2-hydroxyisopropyl) benzene is 1.8:1, by adjusting the jacket hot water flow And the temperature of the high-temperature hot water tank controls the temperature of the jacket hot water bath, and at the same time opens the front and rear valves of the cooling water inlet regulating valve of the inner coil of the condensation kettle, and steadi...
Embodiment 2
[0074] (1) Put 1000 kilograms of toluene into the condensation reactor and start stirring. 572 kilograms of 70wt% TBHP aqueous solutions were dropped into the condensation kettle, 16Kg powdered sodium sulfate was added, the temperature was controlled to be 25°C, stirred for 20 minutes, statically settled for 20 minutes, and the lower aqueous phase (about 154Kg) was separated.
[0075] (2) Put 400 kg of refined DC (98%), 3.5 kg of 50wt% perchloric acid aqueous solution and 3.5 kg of tap water mixture into the condensation reactor at a temperature of about 20°C, wherein DC is a mixture of m- and para-isomers , The ratio of meta-isomers and para-isomers of bis-(2-hydroxyisopropyl)benzene is 2:1. At the same time, open the front and rear valves of the cooling water inlet regulating valve of the inner coil of the condensation kettle, and steadily raise the temperature to about 40°C. When the temperature rises to about 40°C, close the valve on the nitrogen replacement pipe at the t...
Embodiment 3
[0079] (1) 1575Kg of toluene is sucked into the condensation reaction kettle, and the stirring is started. Put 648Kg70wt% TBHP aqueous solution into the condensation kettle, add 26Kg powdered sodium sulfate, control the temperature at 22°C, stir for 25 minutes, settle statically for 20 minutes, and remove the lower aqueous phase (about 178Kg).
[0080] (2) According to the formula, put 450Kg of refined DC (98wt%) into the condensation reaction kettle at a temperature of about 20°C, and then add 4.5Kg of 50% perchloric acid aqueous solution and 4.5Kg of tap water mixture, wherein DC is meta- and para-isomeric Mixtures, the ratio of the meta-isomers of bis-(2-hydroxyisopropyl) benzene to the para-isomers of bis-(2-hydroxyisopropyl) benzene is 1.75:1, and the jacket of the condensation kettle is opened The hot water inlet and outlet valves control the temperature of the hot water bath in the jacket to steadily increase the temperature of the kettle to about 40°C. When the temper...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com