Non-viral gene transfection vector material based on cationic helical peptide
A technology of gene transfection carrier and cation, which is applied in the field of non-viral gene transfection carrier materials and its preparation, can solve the problems of large acidity changes, low efficiency, and many steps, and achieve the effect of low cytotoxicity and high transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1: the synthesis of PALG
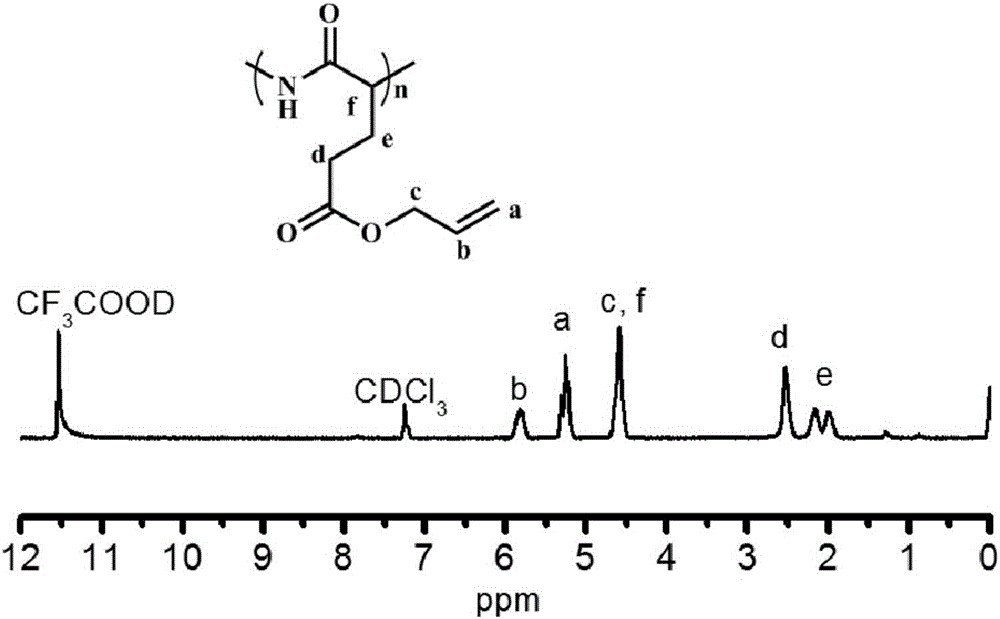
[0072] Weigh 5 g (0.034 mol) of L-glutamic acid and 8.90 g (0.153 mol) of allyl alcohol, mix them uniformly in the flask, and slowly add 4.0 g (0.041 mol) of concentrated sulfuric acid dropwise under ice-bath conditions, and the dropwise addition is completed within 30 minutes. After cooling for 1 h, the ice bath was removed and the temperature was raised to room temperature, and the reaction was continued for 48 h. After the reaction is finished, add enough triethylamine to the system for neutralization, then add enough acetone to stir, and filter out the precipitate. The precipitate was dried overnight in a vacuum oven at room temperature, and the crude product was recrystallized from isopropanol / water, filtered, washed with a sufficient amount of cold acetone, and dried in vacuo. The product γ-allyl-L-glutamate was white flaky crystal, and the yield was 47%.
[0073] Suspend 1.87g (0.01mol) of γ-allyl-L-glutamate in 30mL of anh...
Embodiment 2
[0076] Embodiment 2: the synthesis of PPAETH
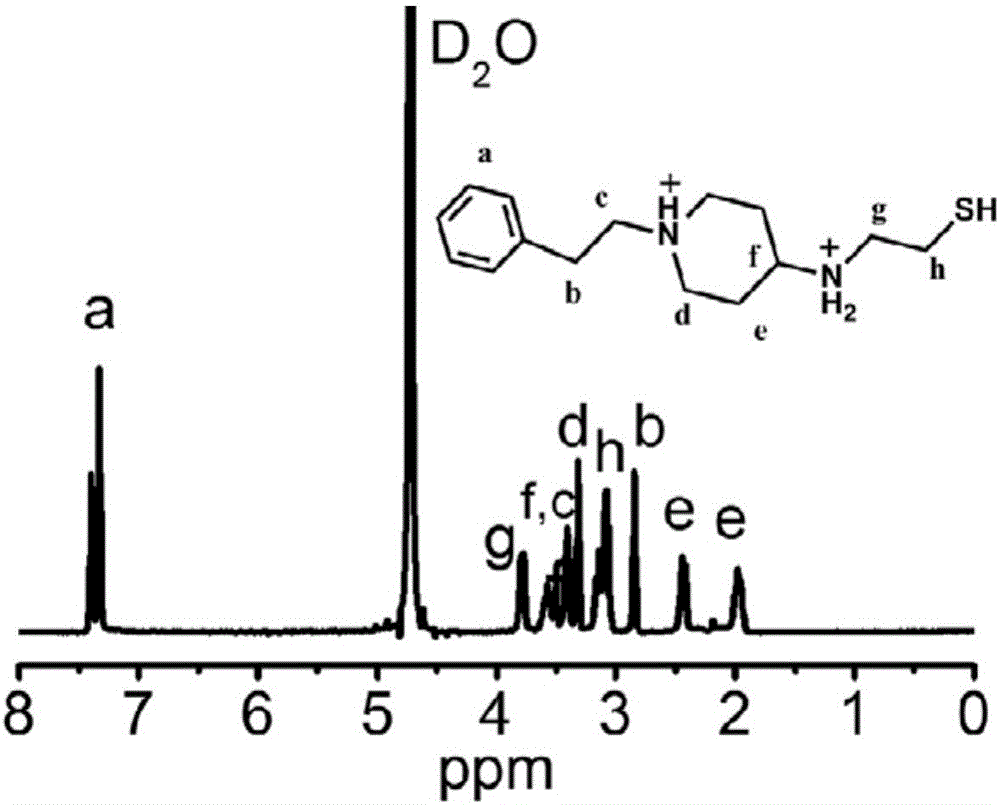
[0077] Dissolve 1.41g (0.01mol) of N-propyl-4-piperidone in 120mL of anhydrous methanol, and then add 1.70g (0.015mol) of cysteamine hydrochloride to fully dissolve it. At this point, add 0.94g (0.015mol) sodium cyanoborohydride, and after it is completely dissolved, add 1.5-2.0mL acetic acid as a catalyst dropwise, and react at room temperature for 72 hours. After the reaction was completed, the solvent was removed by rotary evaporation, quenched by adding 4 mL of concentrated HCl and stirred for 1 h, and most of the water was removed by rotary evaporation. Pour hot ethanol solution into the mixture, filter off the inorganic salt while it is hot, remove ethanol by rotary evaporation, and recrystallize the crude product in methanol. The obtained hydrochloride of thiol-type small molecule amine is white granular, named as PPAETH, and the yield is 34%. 1 H NMR diagram see figure 2 , and its chemical structure is shown in the fol...
Embodiment 3
[0079] Embodiment 3: the synthesis of PPALG
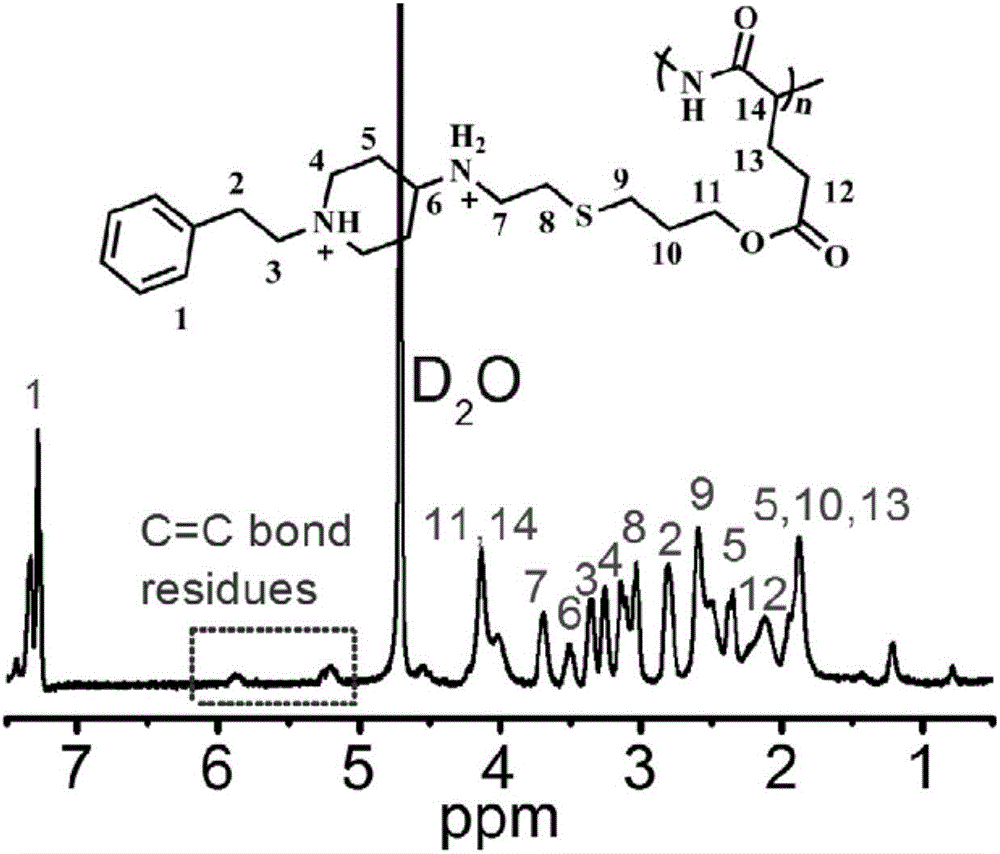
[0080] Weigh 0.1g (0.59mmol C=C bond) of polymer PALG obtained in Example 1 and dissolve it in 3mL DMF to make solution 1; then dissolve 0.18g (0.65mmol-SH) of PPAETH obtained in Example 2 In the least amount of methanol / water mixed solution, make solution two; add photoinitiator Irgacure 651 accounting for 5% of the total mass of all reactants to solution one, and turn on the flashlight Wood Burner's lamp, then slowly add solution 2 dropwise, and the dropwise addition is completed within 1h. Continue to illuminate for 30 minutes, then add 3 mL of DMF, and continue to illuminate for 30 minutes to end the reaction, and dialyze the final mixed solution with a dialysis bag with a molecular weight cut-off of 3500. The distilled water was changed every 4 hours on the first day, and the ethanol solution was changed every 12 hours on the second day to remove the photoinitiator, and then continued to be dialyzed with distilled water for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com