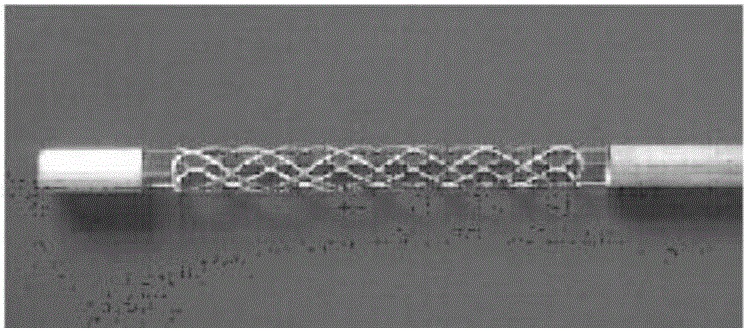
Manufacturing method for manufacturing magnesium alloy vascular stent
A technology for magnesium alloy blood vessels and blood vessel stents, which is applied in the field of processing and preparation of high-purity magnesium alloy absorbable blood vessel stents, can solve the problems of high cost and complicated process, and achieve the effects of reducing manufacturing costs, simplifying processes, and inhibiting stent endothelialization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: prepare high-purity magnesium alloy stent
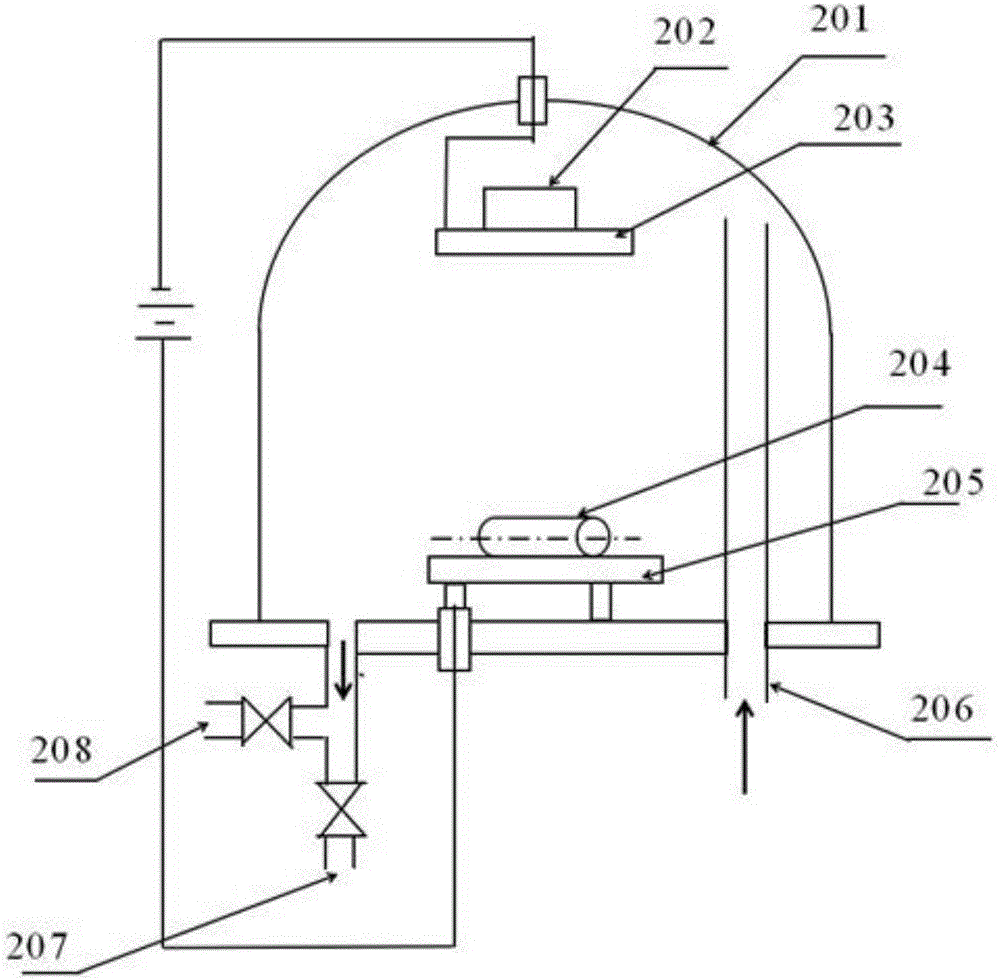
[0042] Such as image 3 As shown, magnetron sputtering equipment (magnetron sputtering deposition system B13-054, machine power 15KW, sputtering power 30W) is used for magnetron sputtering, and magnetron sputtering is carried out in a vacuum chamber 201. Specifically, the prefabricated The body is placed on the anode rotating frame 205 in the vacuum chamber, the magnesium alloy target 202 is secured on the cathode 203 of the vacuum chamber, and then the vacuum chamber 201 is closed. Run mechanical pump 208 to evacuate to 20Pa. Then use the molecular pump 207 to make the vacuum degree of the vacuum chamber reach 5*104Pa. Pass pure argon gas into the vacuum chamber 201 from the gas inlet 206 to make the air pressure in the vacuum chamber 0.8 Pa, and control the rotational speed of the anode rotating frame to 150 r / min.
[0043] Turn on the sputtering power supply, the sputtering power is 20W, so that the magnesi...
Embodiment 2
[0046] Embodiment 2: animal experiment research
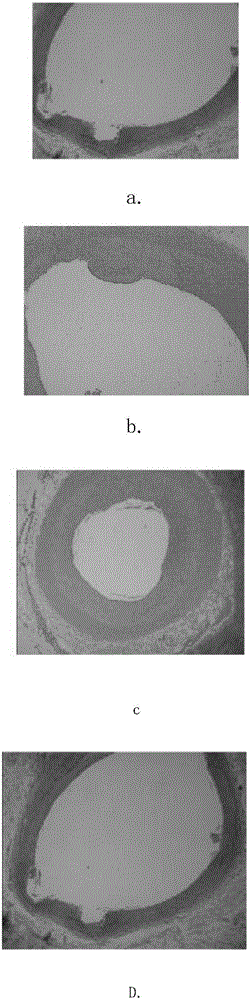
[0047] The research subjects were 50 hybrid test dogs that had been vaccinated against the epidemic. They were randomly divided into 5 groups, with 10 dogs in each group. Each test dog was implanted with an absorbable stent in the femoral artery, and placed 3-5 cm away from the place where the stent was placed. Balloon dilation was performed. 24 hours, three days, five days, one week to six weeks, eight weeks, and three months after the operation, the materials were collected for pathological analysis and tissue biochemical research to clarify the physical and chemical properties, biocompatibility and absorbability of the scaffold. Sex, absorbable time, safety and effectiveness of the stent, and establish a data database. attached image 3 It is the pathological analysis results of the experimental dogs at 1 day, 3 days, 5 days, 1 week, 2 weeks, 3 weeks, 26 days and 4 weeks after operation. The experimental results show that...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com