Preparation method of high-carboxyl-functionality cellulose nanocrystals
A technology of cellulose and functionality, applied in the field of natural polymer chemistry, can solve the problems of limiting the application of cellulose nanocrystals, poor interface interaction, poor dispersion, etc., to improve interfacial compatibility, improve dispersion, and enhance mechanical properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
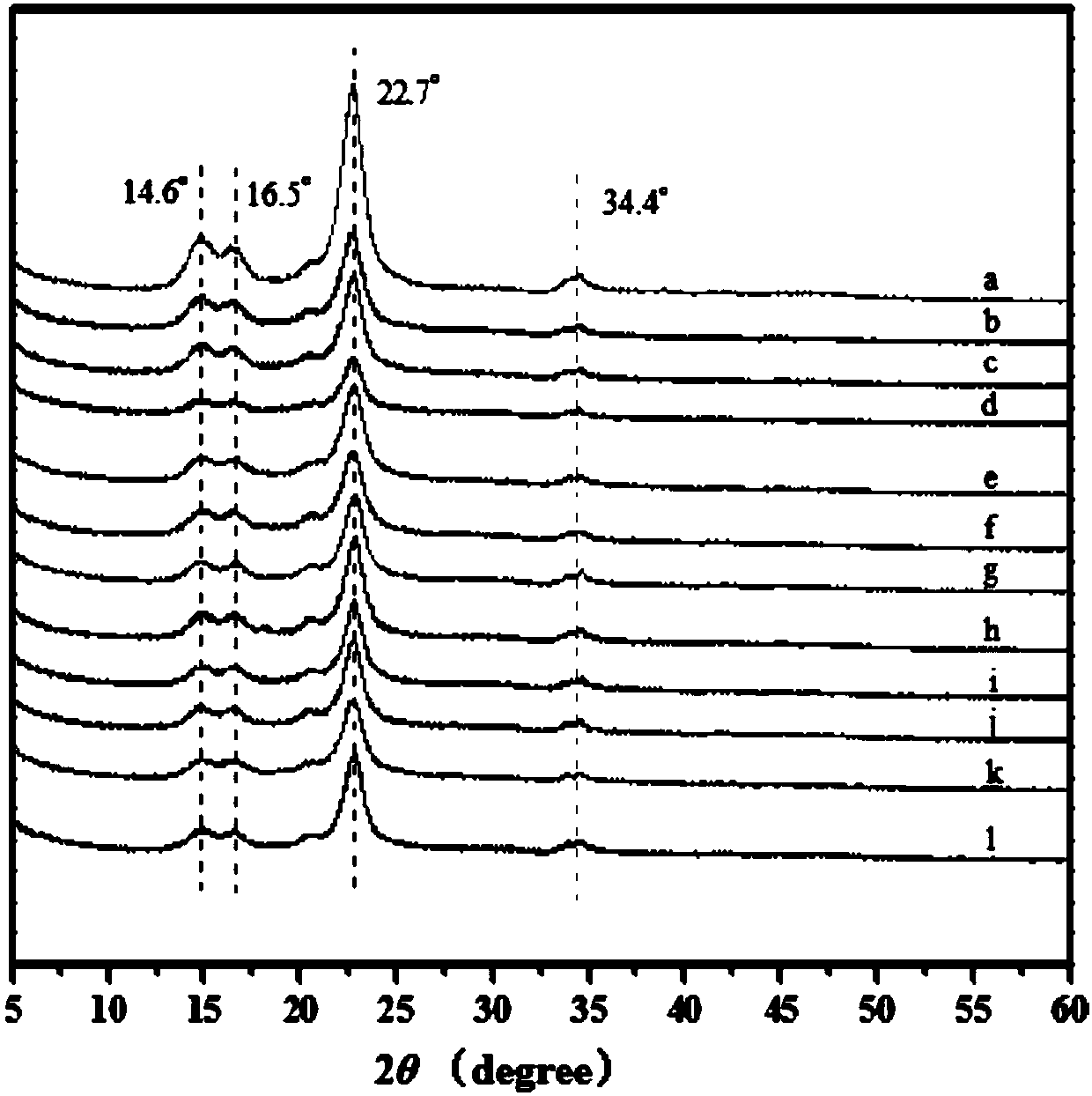
Image
Examples
Embodiment 1
[0032] (1) According to 0.5 parts of hemp fiber, 0 part of 4-diaminopyridine, and 0.5 part of acetic anhydride, select hemp fiber and acetic anhydride, dissolve hemp fiber in 20ml of pyridine, crush the cells for 1min, pour into a three-necked flask, and heat at 75°C , Stir and react for 30 minutes under the protection of nitrogen; dissolve acetic anhydride in 30ml of pyridine, seal with plastic wrap, then ultrasonic for 5 minutes, and stir magnetically at room temperature for 15 minutes, and the acetic anhydride is fully dissolved. In a three-necked flask of cellulose raw material, react under nitrogen protection at 45°C for 5h;
[0033] (2) centrifuge after the end of the reaction, pour off the supernatant, and the precipitated product is successively washed by pyridine, 95% ethanol, distilled water, 2wt% sodium carbonate solution, distilled water, 95% ethanol, and acetone, so as to obtain the surface polycarboxylated fiber cellulose nanocrystals, and then freeze-dried to ob...
Embodiment 2
[0036] (1) According to 0.6 parts of cellulose nanocrystals, 0 parts of 4-diaminopyridine, and 1 part of ethylenediaminetetraacetic dianhydride, select cellulose nanocrystals and ethylenediaminetetraacetic dianhydride, and dissolve the cellulose nanocrystals in In 30ml of pyridine, crush the cells for 2 minutes, pour into a three-necked flask, stir and react for 30 minutes at 75°C under nitrogen protection; dissolve ethylenediaminetetraacetic acid dianhydride in 45ml of pyridine, seal with plastic wrap, then sonicate for 10 minutes, and stir magnetically for 15 minutes at room temperature Finally, the ethylenediaminetetraacetic acid dianhydride was fully dissolved, and the acid anhydride mixture was added dropwise into the three-necked flask containing the cellulose raw material under the protection of nitrogen atmosphere, and reacted for 12 hours under the protection of nitrogen at 55°C;
[0037] (2) centrifuge after the end of the reaction, pour off the supernatant, and the p...
Embodiment 3
[0040] (1) According to 0.7 parts of microcrystalline fiber, 0 parts of potassium carbonate, and 10 parts of diethylenetriaminepentaacetic acid, select microcrystalline fiber and diethylenetriaminepentaacetic acid, and dissolve the microcrystalline fiber in 40ml of nitrogen-nitrogen dimethyl formaldehyde In amide, the cells were crushed for 3 minutes, poured into a three-necked flask, stirred and reacted for 30 minutes at 75°C under nitrogen protection; diethylenetriaminepentaacetic acid was dissolved in 50ml of nitrogen-nitrogen dimethylformamide, sealed with plastic wrap, and then ultrasonicated for 10 minutes. After 15 minutes of magnetic stirring at room temperature, the diethylenetriaminepentaacetic acid was fully dissolved, and the acid anhydride mixture was added dropwise into the three-necked flask containing the cellulose raw material under the atmosphere of nitrogen protection, and reacted under nitrogen protection at 65°C for 24 hours;
[0041] (2) centrifuge after t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com