Simple preparation method of felbinac
A technology of felbinac and ethyl biphenylacetate is applied in the field of easy preparation of felbinac, can solve problems such as industrial application limitation, toxic phosphine and POx smog, and achieves easy operation, short operation period, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of convenient preparation method of felbinac comprises the steps:
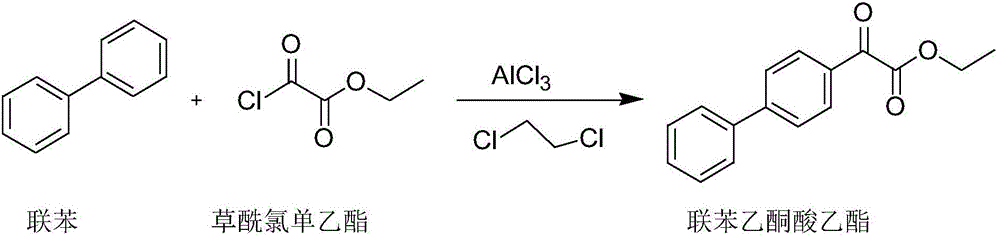
[0034] (1) Preparation of biphenylphenonate
[0035] In a 500mL four-neck flask, under nitrogen protection, add 180ml of dichloroethane, 27.8g (0.18mol) of biphenyl and 36g (0.27mol) of anhydrous aluminum trichloride, stir rapidly and cool down to -5°C, and drop Add 29.5 g (0.25 mol) of monoethyl oxalyl chloride, and control the temperature at -5°C to 5°C during the dropwise addition. After dropping, react at 0°C for 6 hours. After the reaction, pour the reaction solution into concentrated hydrochloric acid / ice water 500mL, V(concentrated hydrochloric acid) / V(ice water)=1:1; Saturated sodium chloride aqueous solution, saturated sodium bicarbonate aqueous solution and purified aqueous solution were washed until neutral, dried over anhydrous sodium sulfate; filtered, and the organic solvent was evaporated to obtain the compound diphenylacetone ethyl ester;
[0036] (2) Synthesis of Felbinac
[0...
Embodiment 2
[0040] A kind of convenient preparation method of felbinac comprises the steps:
[0041] (1) Preparation of biphenylphenonate
[0042] In a 300mL four-neck flask, under nitrogen protection, add 1080ml of dichloroethane, 172.8g (1.08mol) of biphenyl and 230g (1.72mol) of anhydrous aluminum trichloride, stir rapidly and cool down to -4°C, and drop in 4 hours Add 192g (1.62mol) of monoethyl oxalyl chloride, and control the temperature at -3°C to 5°C during the dropwise addition; after the dropwise addition, react at 0°C for 9h, and after the reaction is completed, pour the reaction solution into 3000mL of concentrated hydrochloric acid / ice water, V( Concentrated hydrochloric acid) / V (ice water)=1.2:1; Stir and separate, and the organic phase layer is washed with saturated aqueous sodium chloride solution, saturated aqueous sodium bicarbonate solution and purified aqueous solution until neutral, and dried over anhydrous sodium sulfate; Filter and evaporate the organic solvent to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com