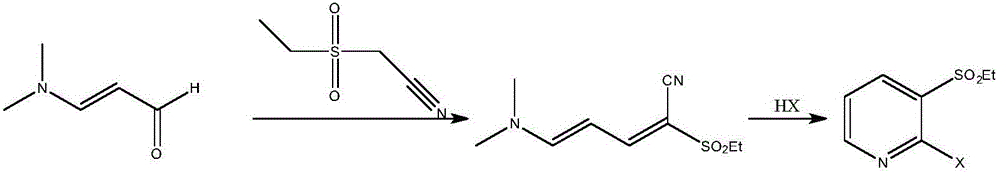
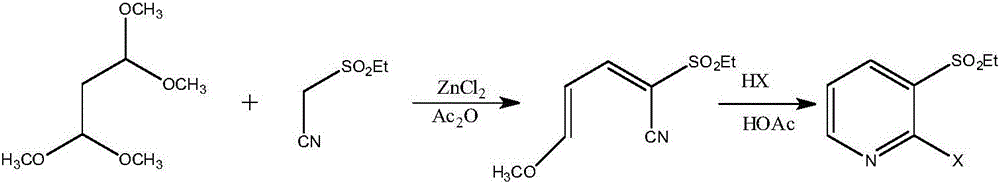
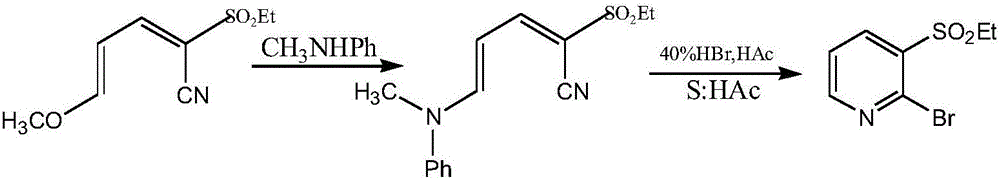
Method for synthesizing 2-hylogen-3-alkyl substituted sulfonyl pyridine and midbody thereof in ultrasonic method
A technology of hydrocarbyl sulfonyl pyridine and hydrocarbyl sulfonyl, which is applied in the field of ultrasonic synthesis of 2-halogenated-3-substituted hydrocarbyl sulfonyl pyridine and its intermediates, and can solve the problem of intractability, low yield, and many "three wastes", etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of 2-methylsulfonyl-5-(N,N-dimethyl)amino-2,4-pentadienenitrile
[0056] In a 500mL three-neck round bottom flask, add 62mL (0.5mol) of 3-dimethylaminoacrolein, 10mL of triethylamine and 59.6g (0.5mol) of methylcyanoethylsulfone, mix well, and prepare the reaction Put the device into the ultrasonic instrument, set the ultrasonic radiation conditions, react under the conditions of temperature 120°C, ultrasonic power 350W and frequency 100KHz, high performance liquid phase tracking and detection of the reaction process until the end of the reaction, add 10mL of deionized water to remove impurities, The crude product was obtained by filtration, and then recrystallized with absolute ethanol to obtain 96.8 g of a light yellow solid with a melting point of 169-171° C. and a product yield of 96.7%. After HR-MS, 1 H NMR, 13 According to CNMR spectrum, the product is 2-methylsulfonyl-5-(N,N-dimethyl)amino-2,4-pentadienenitrile.
[0057] HR-MS of the prod...
Embodiment 2
[0067] Example 2 Preparation of 2-ethylsulfonyl-5-(N,N-diethyl)amino-2,4-pentadienenitrile
[0068] In a 500mL three-necked round-bottomed flask, add 124mL (1.0mol) of 3-diethylaminoacrolein, 10mL of sodium ethoxide and 66.6g (0.5mol) of ethyl cyanoethyl sulfone, mix well, and place the prepared reactor Put it into the ultrasonic instrument, set the ultrasonic radiation conditions, react under the conditions of temperature of 100°C, ultrasonic power of 300W and frequency of 80KHz, TLC detection (petroleum ether: methylene chloride 1:3, sublimation iodine color development) B The reaction of cyanoethyl sulfone was complete, 10 mL of deionized water was added to remove impurities, and the organic phase was distilled to remove the solvent to obtain 116.2 g of a light yellow solid with a melting point of 155-157°C and a yield of 95.9%. The product was characterized by HR-MS, that is, 2-ethylsulfonyl-5-(N,N-diethyl)amino-2,4-pentadienenitrile. HR-MS(ESI):m / zCalcd for C 11 h 18 N...
Embodiment 3
[0069] Example 3 Preparation of 2-isopropylsulfonyl-5-(N,N-diethyl)amino-2,4-pentadienenitrile
[0070] In a 500mL three-neck round bottom flask, add 50mL (0.4mol) of 3-diethylaminoacrolein, 10mL of sodium isopropoxide and 66.6g (0.5mol) of isopropylcyanoethyl sulfone, mix well, and prepare Put the reactor into the ultrasonic instrument, set the ultrasonic radiation conditions, react under the conditions of temperature 80°C, ultrasonic power 300W and frequency 80KHz, TLC detection (petroleum ether: dichloromethane 1:2, sublimation iodine display Color) 3-diethylaminoacrolein was completely reacted, 10 mL of deionized water was added to remove impurities, and the organic phase was distilled to remove the solvent to obtain 97.5 g of light brown oil with a yield of 95.1%. The product was characterized by HR-MS, that is, 2-isopropylsulfonyl-5-(N,N-diethyl)amino-2,4-pentadienenitrile. HR-MS(ESI):m / z Calcd for C 12 h 20 N 2 o 2 S 279.3542[M+Na] + ,found 279.3520[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com