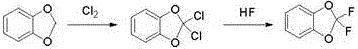
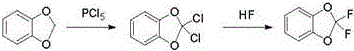
One-pot preparation method of 2,2-difluoro-1,3-benzodioxole
A technology of benzo and PTFE-lined stainless steel, applied in the direction of organic chemistry, can solve problems such as yield decline, high production cost, and difficult reaction control, and achieve the effects of mild production conditions, simple production operation, and improved economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] Below in conjunction with embodiment the present invention is described in further detail.
[0013] Example 1. Add 24.4g (0.2mol) of piperonylcycline, 97.6g of phosphorus oxychloride and 83.3g (0.4mol) of phosphorus pentachloride in a 250ml lined PTFE stainless steel bottle, heat up to reflux reaction, and GC detects the raw material piperonylcycline The content is less than 0.2% and the reaction is over; the reaction solution is subjected to vacuum distillation to distill out the by-product phosphorus trichloride and the solvent phosphorus oxychloride, the temperature of vacuum distillation is 110°C, and the vacuum degree is -0.095MPa; After the end of the distillation, cool to 4°C, add 8 g (0.4 mol) of hydrogen fluoride liquid dropwise at a vacuum of -0.095 MPa, complete the dropwise addition, keep the temperature for 2 hours, then release the by-product hydrogen chloride, then add a saturated aqueous solution of sodium carbonate dropwise to adjust The pH value of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com