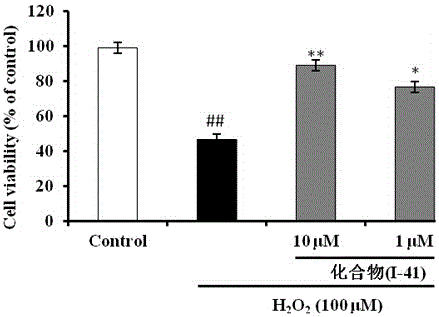
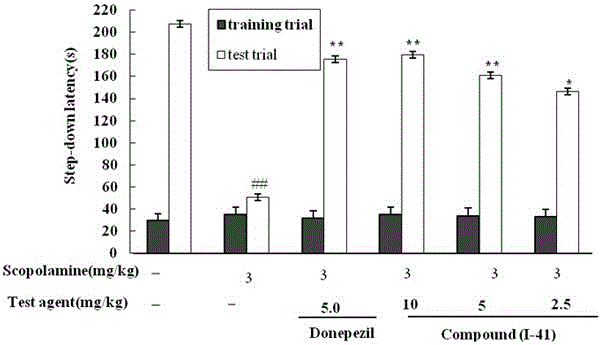
N-(1,2,3,4-tetrahydroisoquinolinyl)-feruloylagmatine-O-alkylamine compound and application
A technology based on tetrahydroisoquinolinyl and tetrahydroisoquinoline, applied in organic chemistry, drug combination, medical preparations containing active ingredients, etc., can solve the problems of neuron loss and deterioration, and achieve low toxicity and inhibition Aggregation activity, good effect in treating Alzheimer's disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
[0039] N -(1,2,3,4-Tetrahydroisoquinolinyl)-ferulamide- O -Alkylamine compound (I), obtained by the following preparation method:
[0040] ,
[0041] In the formula: n is 2~12, Y represents H, methoxy, C 1 ~C 12 Alkyl, halogen or dimethylamino, with substituents at any possible position on the benzene ring; NR 1 R 2 represents 1,2,3,4-tetrahydroisoquinoline, benzylpiperidine, benzylmethylamine or 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline;
[0042] Step (i): Add ferulic acid 1, condensing agent and solvent into the reaction flask, stir evenly, then add 1,2,3,4-tetrahydroisoquinoline compound 2, after the addition, the temperature T 1 under stirring reaction n 1 hours, TLC monitoring; after the reaction, the solvent was evaporated under reduced pressure, water was added to the residue, extracted with dichloromethane, the organic layers were combined and washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, filtered, and the filt...
Embodiment 16
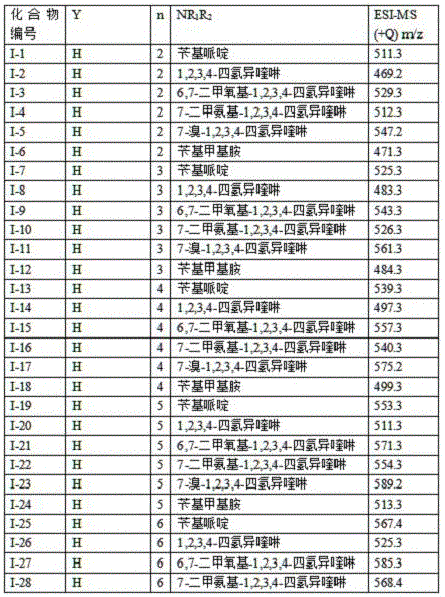
[0060] The specific process conditions are the same as in Example 1, the difference is: investigate different substituents, the specific substituents are shown in Table 4, and the obtained N -(1,2,3,4-Tetrahydroisoquinolinyl)-ferulamide- O - Alkylamine compound (I), the chemical structure of which is confirmed by 1H-NMR, 13C-NMR and ESI-MS.
[0061] Experimental results of different substituents N -(1,2,3,4-Tetrahydroisoquinolinyl)-ferulamide- O -Alkylamine compounds (I) are shown in Table 4.
[0062] Table 4 Experimental results of different substituents
[0063]
[0064] .
Embodiment 17
[0066] N -(1,2,3,4-Tetrahydroisoquinolinyl)-ferulamide- O -The preparation method of alkylamine compound (I) and acid salt formation, comprising the following steps:
[0067] In reaction bottle, add embodiment 1 gained N -(1,2,3,4-Tetrahydroisoquinolinyl)-ferulamide- O - Alkylamine compound (I) 2.5 mmol and acetone 50 mL, after stirring evenly, add 10.0 mmol of a suitable acid, heat and reflux and stir for 30 minutes, cool to room temperature after the reaction, evaporate the solvent under reduced pressure, and wash the residue with acetone Recrystallize, and filter the precipitated solid to obtain N -(1,2,3,4-Tetrahydroisoquinolinyl)-ferulamide- O - the salt of alkylamine compound (I), its chemical structure is verified 1 Confirmed by H NMR and ESI-MS.
[0068] Described acid is selected from hydrochloric acid, hydrobromic acid, nitric acid, sulfuric acid, phosphoric acid, C 1-6 Fatty carboxylic acids (such as: formic acid, acetic acid, propionic acid, etc.), oxalic ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com