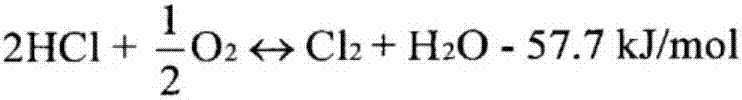Preparation method of catalyst for oxidization of hydrogen chloride for chlorine production
A catalyst, oxidation technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, preparation with chloride, physical/chemical process catalyst, etc., can solve the conditions that fail to reach large-scale industrialization, sodium hydroxide Overcapacity, unfavorable large-scale industrial promotion, etc., to achieve the effect of good reaction effect, low price, improved activity and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
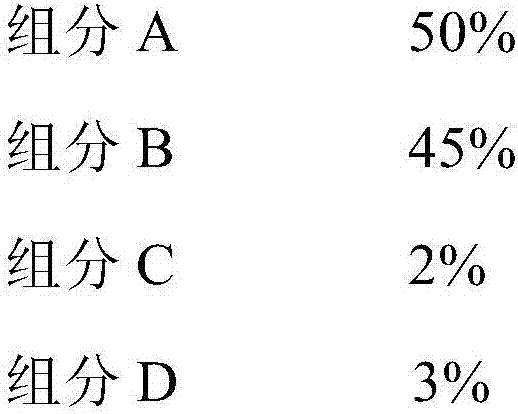
[0045] A certain amount of catalyzer is loaded into the fixed-bed tubular reactor, and the oxidation of hydrogen chloride to chlorine catalyst precursor is implemented according to the following weight percentages:
[0046]
[0047] Component A is copper oxide, component B is chromium oxide, component C is potassium chloride, and component D is silicon oxide. The obtained hydrogen chloride oxidation to chlorine catalyst is marked as A.
[0048] The catalyst for producing chlorine by oxidation of hydrogen chloride of the present invention is prepared by the following method.
[0049] a) Component A copper oxide is uniformly mixed with component B chromium oxide, put into a mortar and grind for 15 minutes, and dry;
[0050] b) Add the metal oxide powder obtained in step a) to an aqueous solution containing component C potassium chloride (1mol / L), the mass ratio of the metal oxide powder to the aqueous solution is 1:5, heat to reflux, and stir for 2 hours , filtered, washed,...
Embodiment 2
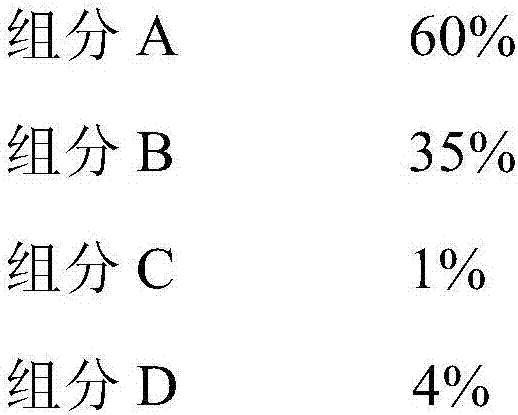
[0055] The hydrogen chloride oxidation of the present invention produces chlorine catalyst precursor according to following weight percent implementation:
[0056]
[0057] Component A is copper oxide, component B is chromium oxide, component C is potassium chloride, and component D is silicon oxide. The resulting hydrogen chloride oxidation to chlorine catalyst is marked as B.
[0058] The catalyst for producing chlorine by oxidation of hydrogen chloride of the present invention is prepared by the following method.
[0059] a) Component A copper oxide is uniformly mixed with component B chromium oxide, put into a mortar and grind for 15 minutes, and dry;
[0060] b) Add the metal oxide powder obtained in step a) into an aqueous solution containing component C potassium chloride (0.5mol / L), the mass ratio of the metal oxide powder to the aqueous solution is 1:5, heat to reflux, and stir to react 2h, filter, wash, dry, and roast at 350°C for 6h;
[0061] c) adding the mat...
Embodiment 3
[0070] The hydrogen chloride oxidation of the present invention produces chlorine catalyst precursor according to following weight percent implementation:
[0071]
[0072] Component A is copper oxide, component B is chromium oxide, component C is potassium chloride, and component D is silicon oxide. The resulting hydrogen chloride oxidation to chlorine catalyst is marked as D.
[0073] The preparation steps of the catalyst for hydrogen chloride oxidation to chlorine in the present invention are the same as in Example 1, the difference is the content of each component.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com