A kind of andrographolide-19-o-glucoside and its preparation method and application in the preparation of anti-inflammatory drugs
A technology of andrographolide and glucoside, which is applied in the direction of anti-inflammatory agents, drug combinations, antipyretics, etc., can solve the problems of poor stereo and regioselectivity, residual chemical substances, easy to produce by-products, etc., and achieve stereo and regioselectivity The effect of good sex, high purity and good medicinal prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
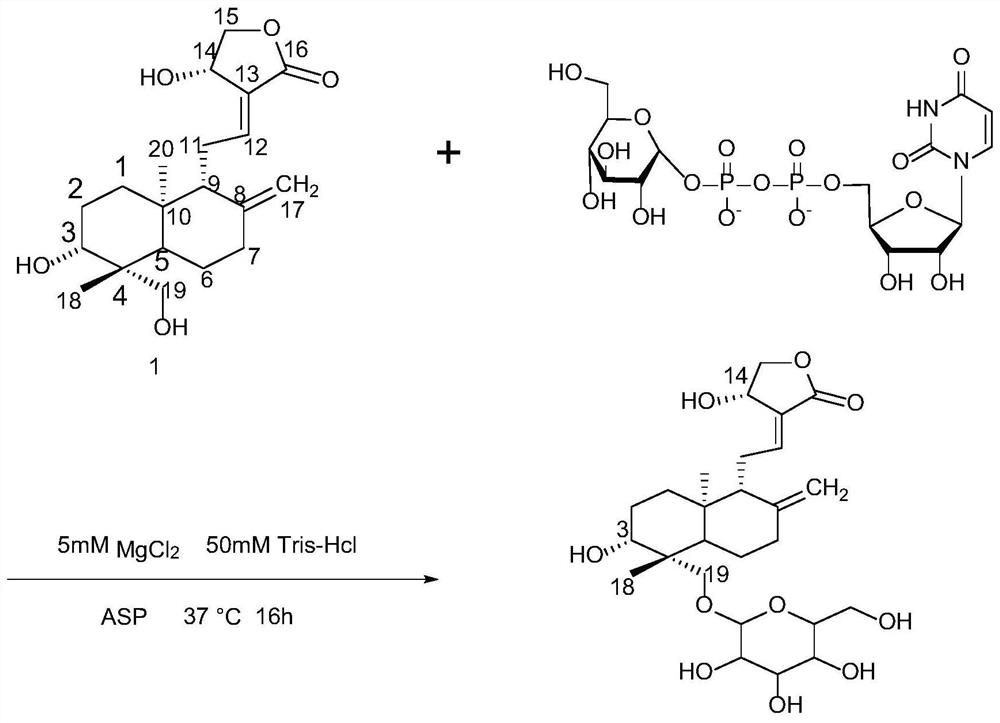
[0042]1.75g of andrographolide, 25mM of uridine-5'-diphosphate glucose sodium salt (UDPG), 2g of ASP (A242V / S132F / P67T) glycosyltransferase ( The preparation method refers to the literature Williams, Gavin J; Zhang, Changsheng; Thorson, Jon S; Expanding the promiscuity of a natural-product glycosyltransferase by directed evolution; Nat Chem Biol, 2007, 3, 657-662) and 5mM MgCl 2 , 50mM Tris-HCl (pH 8.0) mixed, placed in an incubator at 37°C, reacted for 16h, centrifuged at 12000rpm for 15min, concentrated the supernatant, put the mixture on a macroporous resin column, and eluted with pure water for 2 retention volumes , remove water-soluble impurities, then use 50% ethanol to elute 2 retention volumes, after 50% ethanol eluate is concentrated, promptly obtain andrographolide-19-O-glucoside (productivity 80%), biosynthesis roadmap see figure 1 .
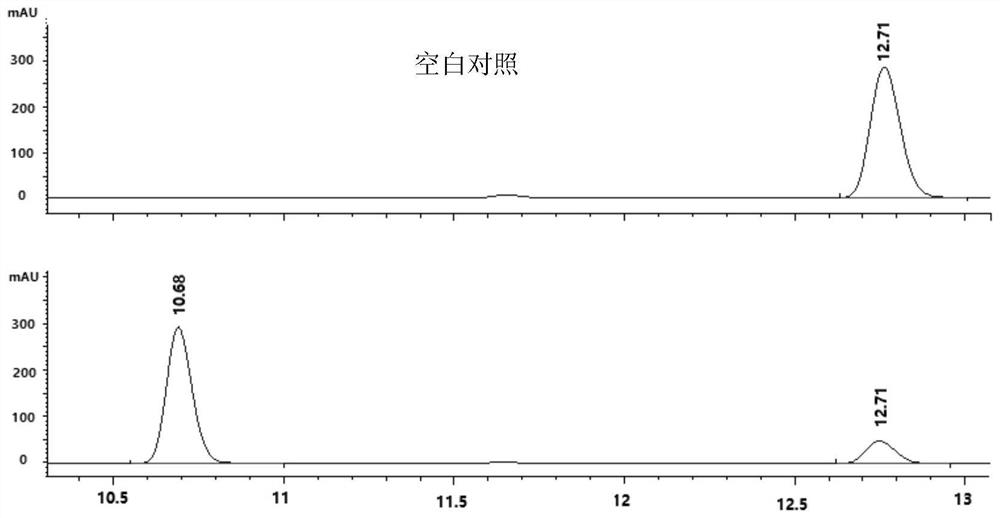
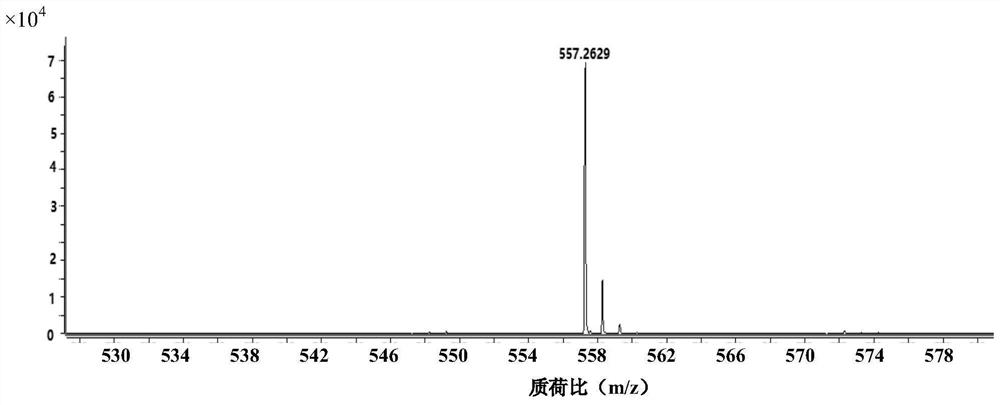
[0043] The characterization diagram of the obtained product is shown in Figure 2 to Figure 5 , according to the high-resolutio...
Embodiment 2
[0047] 1.75g of andrographolide, 50mM uridine-5'-diphosphoglucose sodium salt (UDPG), 2g of ASP(A242V / S132F / P67T) glycosyltransferase expressed in Escherichia coli BL21(DE3)PLysS and 5mM MgCl 2 , 50mM Tris-HCl (pH 8.0) mixed, placed in an incubator at 37°C, reacted for 16h, centrifuged at 12000rpm for 15min, concentrated the supernatant, put the mixture on a macroporous resin column, and eluted with pure water for 2 retention volumes , remove water-soluble impurities, and then use 50% ethanol to elute 2 retention volumes, and after the 50% ethanol eluate is concentrated, andrographolide-19-O-glucoside (yield 99%) is obtained.
Embodiment 3
[0049] 1.75g of andrographolide, 25mM of uridine-5'-diphosphate glucose sodium salt (UDPG), 2g of ASP (A242V / S132F / P67T) glycosyltransferase derived from the expression of Escherichia coli BL21 (DE3) PlysS and 5mM MgCl 2 , 50mM Tris-HCl (pH 6.0) mixed, placed in an incubator at 37°C, reacted for 16h, centrifuged at 12000rpm for 15min, concentrated the supernatant, put the mixture on a macroporous resin column, and eluted two retained volume, remove water-soluble impurities, then use 50% ethanol to elute 2 retention volumes, after 50% ethanol eluate is concentrated, obtain white powder, be andrographolide-19-O-glucose (productivity 37% ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com