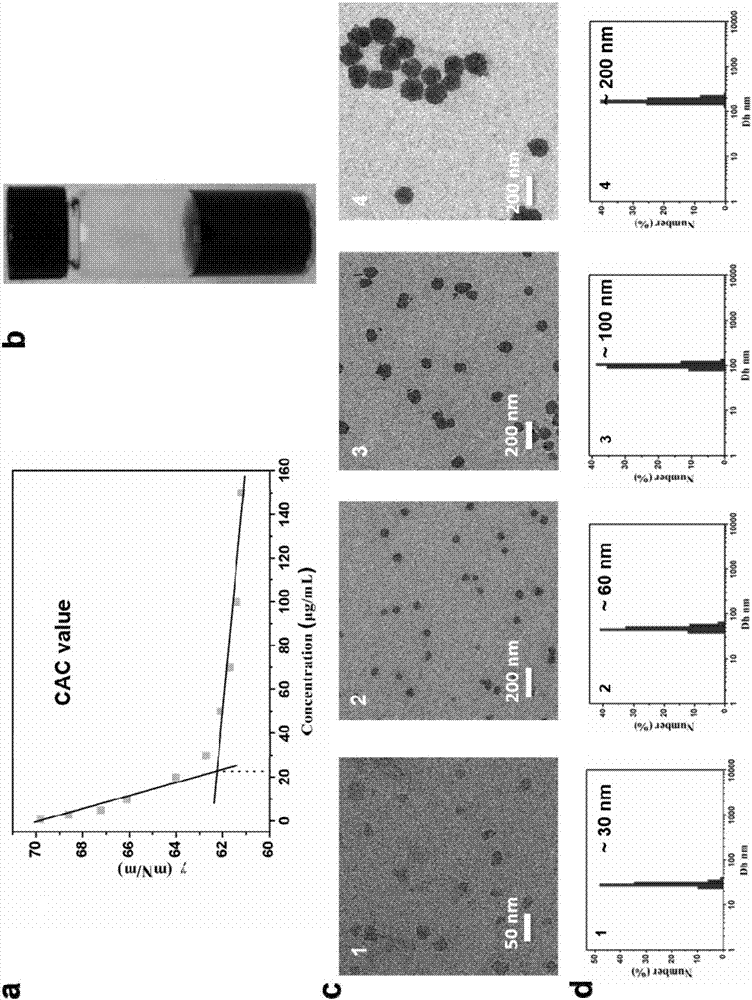
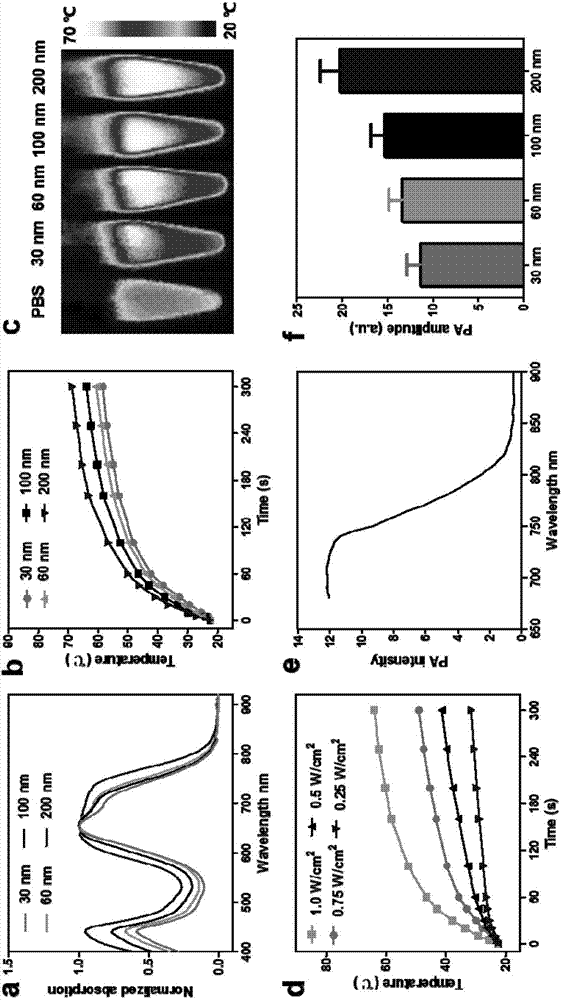
Perylene bisimide multifunctional nano particles with adjustable size and preparation method and application thereof
A perylene imide and nanoparticle technology, which is applied in the field of preparation of multifunctional pure organic peryleneimide nanoparticles, can solve the problems of limited and restricted biological applications, and achieve uniform appearance, low price and simple synthesis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
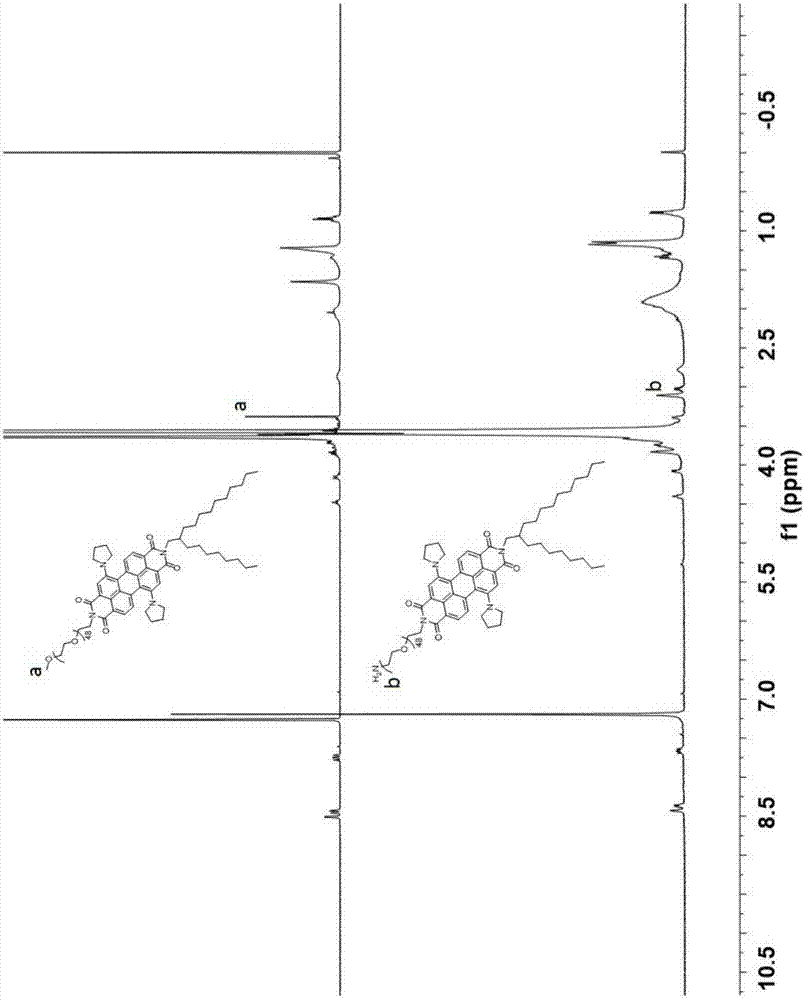
[0049] Under nitrogen protection, dibromoperylene tetraanhydride (0.85g, 1.55mmol) was dispersed in 100mL of methylpyrrolidone, and then C20 primary amine (1.53g, 5.1mmol) with dovetail structure was added, acetic acid (5g, 8.33mmol ). The reaction was stirred at 85°C for 8 hours. After the reaction, it was cooled to room temperature, and poured into the reaction system into a 1N aqueous solution of hydrochloric acid, and a large amount of red precipitates could be seen to be precipitated at the same time. The red precipitate was filtered and washed with plenty of water. The crude product is obtained after drying. The obtained crude product was passed through the column with 200-300 mesh silica gel as the stationary phase and petroleum ether / dichloromethane (volume ratio 1:4) as the eluent to obtain 1.6 g of red solid with a yield of 90%. 1 H NMR (400MHz, CDCl 3 ):δ=9.50(m,2H),8.94(d,2H),8.71(s,2H),4.26–4.19(m,4H),1.79–1.70(m,4H),1.50–1.43(m,66H ),1.00(t,12H)p...
Embodiment 2
[0051]
[0052] By substituting the dibromine at the harbor position of peryleneimide with dipyrrole, the peryleneimide molecule with near-infrared absorption can be obtained. Dibromoperylene (331.8mg, 0.3mmol) and 8mL of pyrrolidine were heated to 55°C and stirred for 24 hours under the protection of the monomer. After the reaction was finished, excess pyrrolidine was removed by a rotary evaporator. The crude product was passed through a column using 200-300 mesh silica gel as a stationary phase and pure dichloromethane as an eluent to obtain 248 mg of a green solid with a yield of 75%. In this step, the isomers of peryleneimide, the isomers of 1, 6 and 1, 7 positions can be distinguished by carefully passing through the column, and the isomers with better near-infrared absorption can be obtained. Molecular structure of 1,7 positions. 1 H NMR (400MHz, CDCl 3 ):δ=8.28(d,4H),7.53(s,4H),4.25–4.19(t,4H),3.67(m,4H),2.65–2.51(m,4H),2.03–1.72(m,12H ),1.48(m,66H),1.00(t,12H)pp...
Embodiment 3
[0054]
[0055] In order to prepare an asymmetric peryleneimide molecular structure, one end of the alkyl chain at the imide site is removed. The symmetrical molecule (1.09g, 1.00mmol) obtained in the previous step and sodium hydroxide (4.68g, 83.40mmol) were added to 36mL of isopropanol solution, and heated to reflux under nitrogen protection for 0.5 hours. The reaction perylene was introduced into 50 mL of acetic acid solution. After stirring overnight, the precipitated green solid was filtered and washed with copious amounts of water and methanol. The obtained crude product was passed through a column using 200-300 mesh silica gel as a stationary phase and pure dichloromethane as an eluent to obtain an asymmetric product (0.60 g, 70%). 1 H NMR (400MHz, CDCl 3 )8.45-8.32(m,4H),7.46-7.53(d,2H),4.22(d,2H),3.85-3.65(m,4H),2.85-2.65(m,4H),2.25-1.95(m, 8H),1.48(m,33H),0.80(t,6H)ppm.HRMS:calcd.for C 52 h 63 N 3 o 5 [M+H]+809.4876; found 809.4844.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com