Activated neutrophil membrane coated neutrophil like nanoparticles and preparation method
A bionic nanometer and cell membrane technology, applied in the field of neutrophil-like nanocarriers and its preparation, can solve the problems of poor prognosis, achieve low immunogenicity, high safety and medical transformation prospects, biocompatibility and High safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
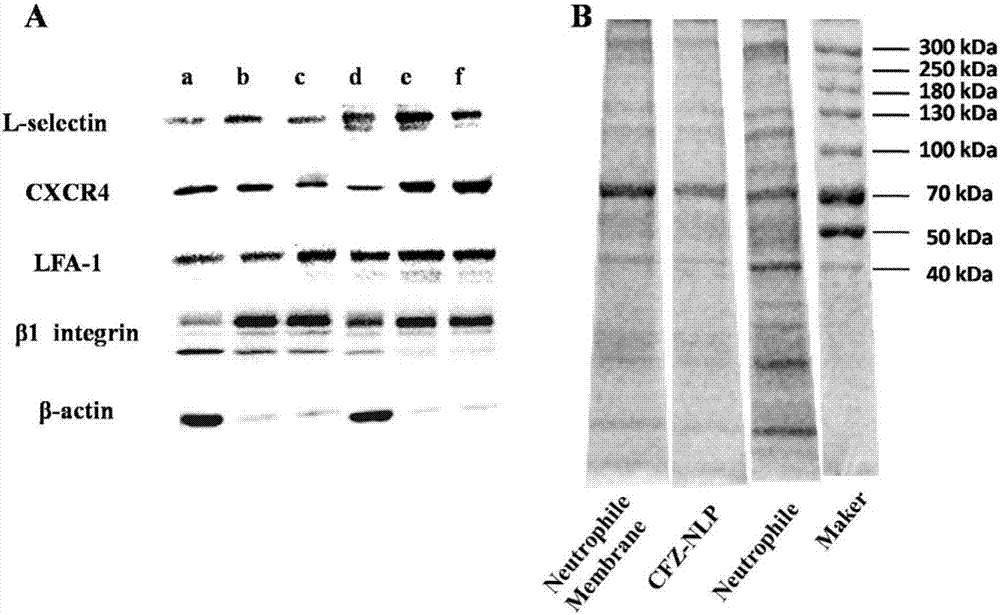
[0045] Preparation and Characterization of the Activated Neutrophil Membrane of Example 1
[0046] After mice were anesthetized with 5% chloral hydrate, blood was taken from the heart into EP tubes added with heparin. Take a 50ml centrifuge tube and carefully add 15ml mouse peripheral blood neutrophil separation solution. Carefully draw the blood sample with a straw, add it to the liquid surface of the separation liquid, and centrifuge at 500g for 25min. After centrifugation, two ring-shaped milky white cell layers will appear in the centrifuge tube, the upper layer of cells is a mononuclear cell layer, and the lower layer of cells is a neutrophil layer. Carefully absorb the neutrophil layer in the separation solution with a pipette, add 3 times the volume of red blood cell lysate, mix gently by pipetting, lyse for 10 minutes, centrifuge at 300g for 10 minutes, and discard the red supernatant. Repeat the lysis step once to obtain neutrophils. Add 10ml of PBS to the obtained...
Embodiment 2
[0051] Example 2 Preparation and Characterization of Activated Neutrophil Membrane-Coated Carfilzomib Nano-Drug Delivery System
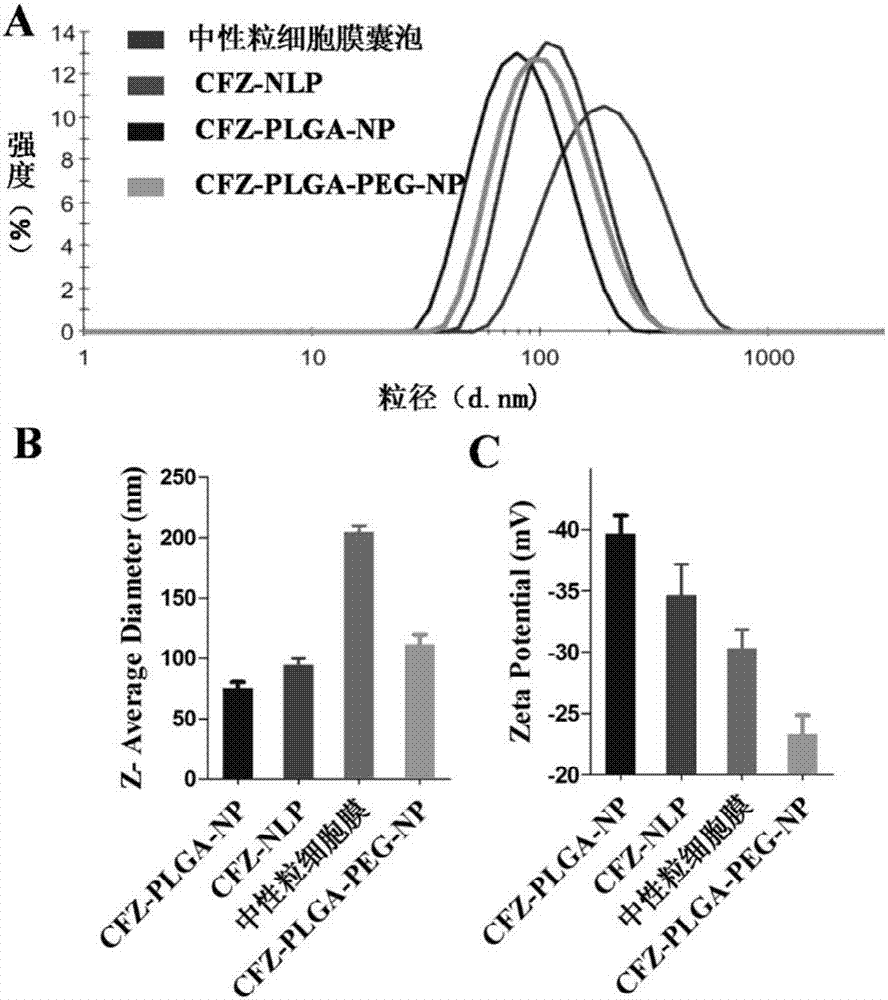
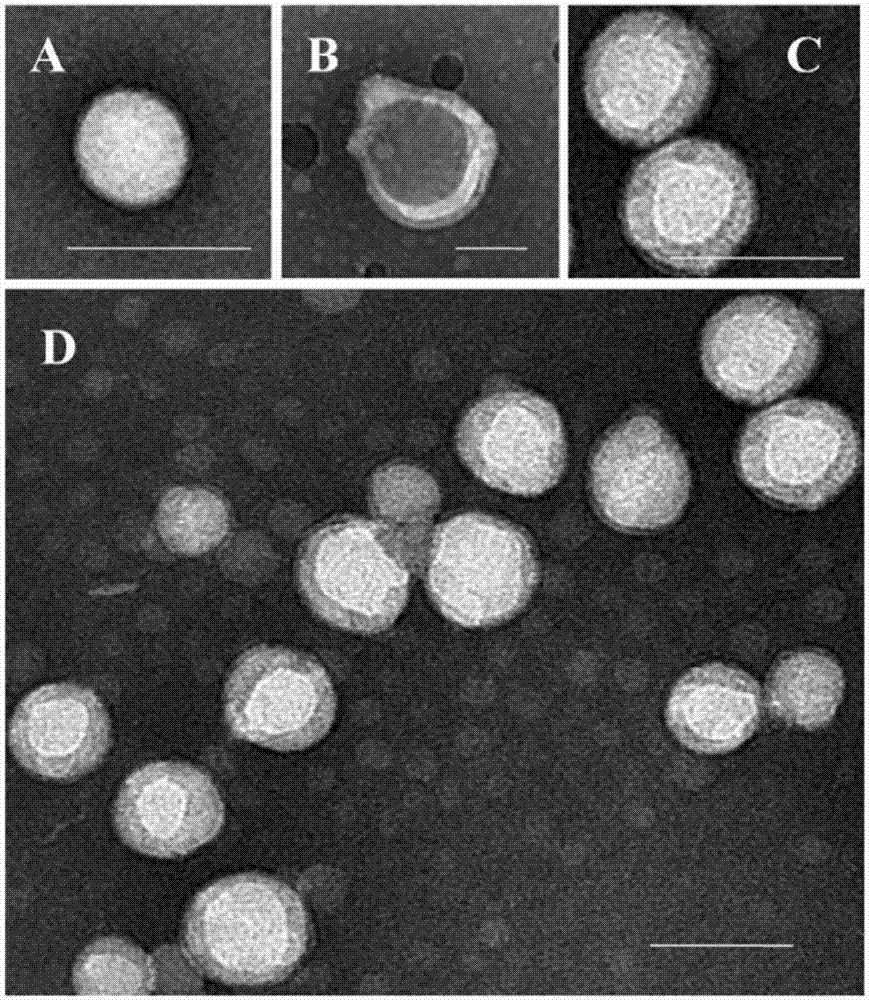
[0052] Accurately weigh 10 mg of PLGA polymer material, add 1 mL of carfilzomib dichloromethane solution to dissolve into organic phase, add 2 mL of 1.0% sodium cholate aqueous phase solution, 240w, 50s ultrasonic emulsification, and then immediately pour into a certain Measure 0.5% sodium cholate solution, magnetically stir for 5 minutes, exhaust the organic solvent by rotary evaporation, and centrifuge at low temperature and high speed (14500rpm, 4°C, 60min) to obtain carfilzomib-loaded NP nanoparticles (CFZ-NP for short);
[0053] Precisely weigh the neutrophil membrane, use the mini-type Avanti liposome / nano extruder, repeatedly pass through the polycarbonate membrane with a pore size of 200nm to aggregate into small lipid vesicles in the aqueous phase, and the mass ratio is The nanoparticles prepared by the PLGA material of 1:1 are evenly mixed...
Embodiment 3
[0056] Qualitative and Quantitative Research of Example 3 NLP Uptake on Static and Flowing 4T1 Cells
[0057] qualitative analysis
[0058] a. Incubation process in static state: 2×10 per well 4 The density of 4T1 cells was inoculated in a 24-well culture plate, incubated in an incubator for 24 h to adhere to the wall, and then the culture medium was sucked off, and NLP and NP (nanoparticle concentration: 400 μg mL) were added with 0.1% coumarin-6 -1 ), fixed with 4% paraformaldehyde after incubation for 2 hours, and stained the nucleus with Hoechst;
[0059] b. Incubation process in a flowing state: Brookfield cone-plate viscometer is used to generate a certain shear force, the circulating water bath ensures a constant temperature of 37°C, and the 4T1 cell suspension is prepared according to 5×10 4 Add 500 μL of samples / mL into the sample pool, and then add 500 μL of NLP and NP loaded with 0.1% coumarin-6 (nanoparticle concentration 800 μg·mL -1 ), at 188s -1 After incuba...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com