Malondialdehyde fluorescence probe, and preparation method and application thereof
A technology of fluorescent probe and malondialdehyde, which is applied in the field of analytical chemistry, can solve the problems such as non-destructive detection of malondialdehyde, and achieve the effect of high yield, simple synthesis, and detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Synthesis of compound 3:
[0032]
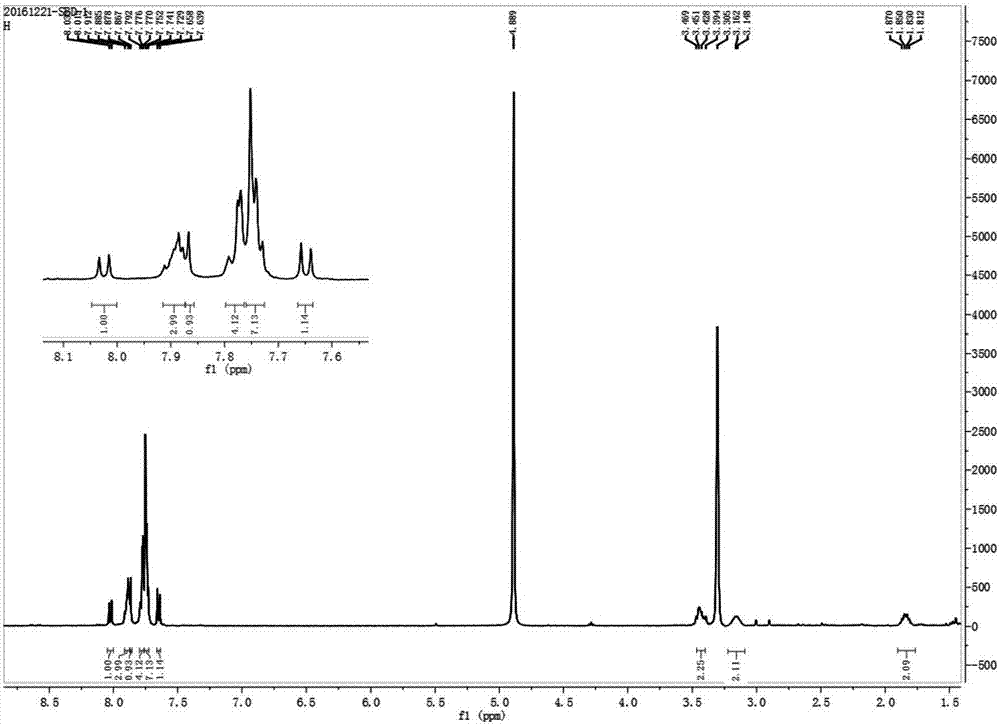
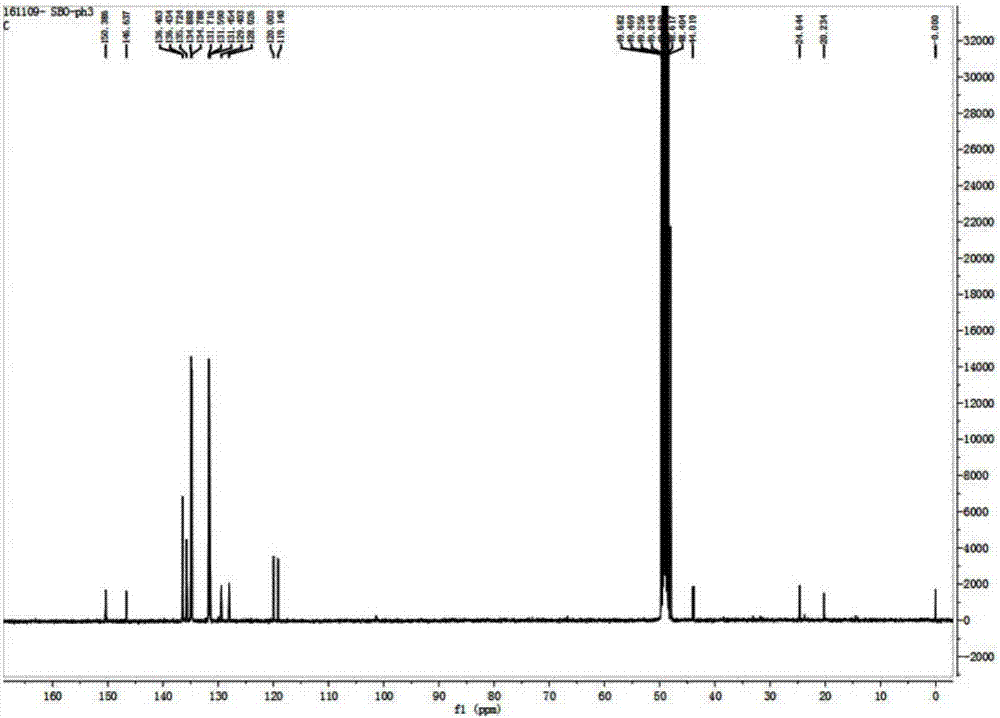
[0033] Will take 50mg 4-chloro-7-chlorosulfonyl-2,1,3-benzoxadiazole (1) (0.2mmol) and 80mg 3-propylaminotriphenylphosphine bromide (2) (0.2mmol ) As in the round bottom flask, add dichloromethane to make it dissolve completely, then dropwise add a drop of triethylamine, stir under nitrogen protection and normal temperature for 1 hour, after the reaction is completed, the solvent (dichloromethane) is distilled under reduced pressure by a rotary evaporator Removal gave the crude product. Using ethyl acetate and petroleum ether at a volume ratio of 2:1 as the eluent, the silica gel (200-300 mesh) chromatography column was used for purification to obtain 79 mg of light yellow solid 3 (yield: 63%). 1 H NMR (400MHz, CD 3 OD),δ(ppm):1.81-1.87(m,2H),3.15-3.16(2H),3.39-3.47(2H),7.64-7.66(d,J=7.6Hz,1H),7.73-7.75(7H ),7.77-7.79(4H),7.87(s,1H),7.88-7.91(3H),8.01-8.03(d,J=7.6Hz,1H); 13 C NMR (100MHz, CD 3 OD): δ (ppm): 20.23, 24.64, 44.02...
Embodiment 2
[0038] Fluorescence Spectrum Changes of Probe FDP Reacted with Different Concentrations of Malondialdehyde
[0039] The probe FDP prepared in Example 1 was dissolved in ethanol to make a probe mother solution with a concentration of 1 mM, and 220 mg of 1,1,3,3-tetraethoxypropane was dissolved in 80 mL of aqueous hydrochloric acid with a concentration of 10 mM. Heated at 50°C for 1 hour, cooled to room temperature, and then diluted to 100 mL with aqueous hydrochloric acid to obtain a mother liquor of malondialdehyde with a concentration of 100 mM. Take out 30 μ L from the probe mother liquor and join in the centrifuge tube of 5mL, add the malondialdehyde mother liquor of different equivalents (0-120eq) (the described equivalence refers to the material amount of malondialdehyde in the malondialdehyde mother liquor relative to the probe The multiple of the amount of substance of the probe in the mother liquor), diluted to 3mL with different volumes of PBS aqueous solution (concen...
Embodiment 3
[0041] Fluorescence changes of probe FDP and malondialdehyde with time
[0042] Take 30 μL from the fluorescent probe mother solution in Example 2 and add it to a 5mL centrifuge tube, add 30 μL of malondialdehyde mother solution with a concentration of 100mmol / L, and then dilute to 3mL with 2.94mL PBS buffer solution (concentration 25mM, pH 7.4) , prepared as a test solution with a probe concentration of 10 μmol / L and containing 1% ethanol. With an excitation wavelength of 373nm, its fluorescence spectrum changing with time was tested. Depend on Figure 7 It can be seen that as time increases, the fluorescence intensity at 554 nm gradually increases, and reaches the maximum value at about 15 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com