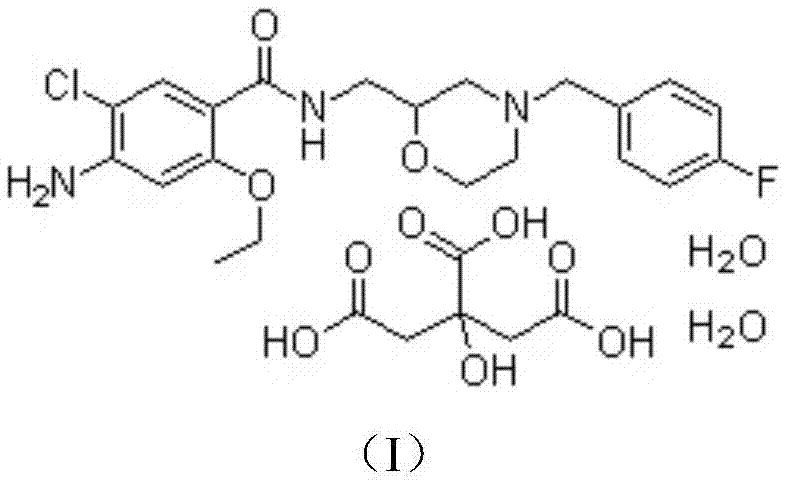
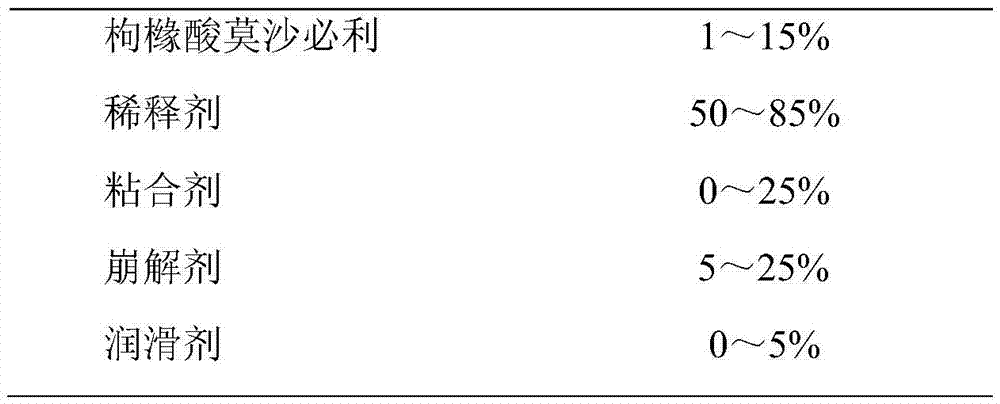
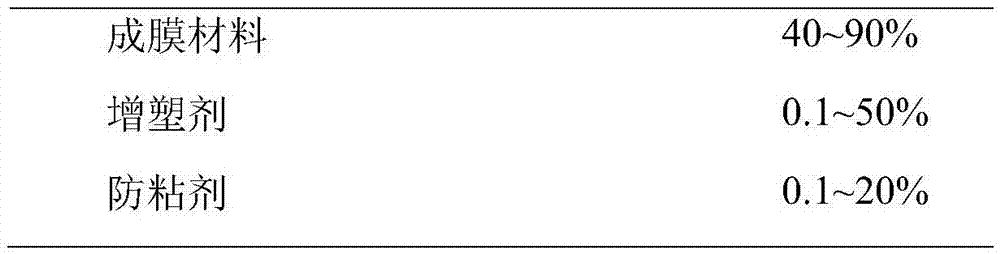
Medicine composition of mosapride citrate and preparation method of same
A technology of mosapride citrate and a composition is applied in the field of pharmaceutical compositions containing mosapride citrate, and achieves the effects of good thermal stability and good masking of bitter taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1 (sample 4)
[0077] [Table 7] Chip composition
[0078]
[0079]
[0080] ▲When necessary, use lactose as an adjusting component to change tablet weight.
[0081] [Table 8] Composition of film coating aqueous solution
[0082]
[0083] ▲Purified water accounts for about 90% (w / w) of the film coating aqueous solution.
[0084] The plain tablet (core tablet) containing mosapride citrate was prepared according to the composition ratio shown in the above table [7].
[0085] Dissolve 10 g of hypromellose in 40 g of purified water to obtain a binder solution. The prepared binder solution was added to the mixture containing mosapride citrate (5.29g), lactose (51.91g), starch (32.4g) and low-substituted hydroxypropyl cellulose (9.5g). Granulated by method, dried and sieved through a 30-mesh sieve, and blended with low-substituted hydroxypropyl cellulose (9g), magnesium stearate (1.3g) and silicon dioxide (0.6g). Then the obtained granules are pressed ...
Embodiment 2
[0087] Embodiment 2 (sample 5)
[0088] [Table 9] Chip composition
[0089]
[0090] ▲When necessary, use lactose as an adjusting component to change tablet weight.
[0091] [Table 10] Composition of film coating aqueous solution
[0092]
[0093] ▲Purified water accounts for about 90% (w / w) of the film coating aqueous solution.
[0094] The plain tablet (core tablet) containing mosapride citrate was prepared according to the composition ratio shown in the above table [9].
[0095] 15 g of hypromellose was dissolved in 40 g of purified water to obtain a binder solution. The prepared binder solution was added to the wet mixture containing mosapride citrate (5.29g), lactose (46.91g), starch (32.4g) and low-substituted hydroxypropyl cellulose (9.5g) Granulated by method, dried and sieved through a 30-mesh sieve, and blended with low-substituted hydroxypropyl cellulose (9g), magnesium stearate (1.3g) and silicon dioxide (0.6g). Then the obtained granules are pressed int...
Embodiment 3
[0097] Embodiment 3 (sample 6)
[0098] [Table 11] Chip composition
[0099]
[0100] ▲When necessary, use lactose as an adjusting component to change tablet weight.
[0101] [Table 12] Composition of film coating aqueous solution
[0102]
[0103] ▲Purified water accounts for about 90% (w / w) of the film coating aqueous solution.
[0104] The plain tablet (core tablet) containing mosapride citrate was prepared according to the composition ratio shown in the above table [11].
[0105] 45g of purified water was added to the mixture containing mosapride citrate (5.29g), lactose (61.91g), starch (32.4g) and low-substituted hydroxypropyl cellulose (9.5g) for wet granulation, After drying, pass through a 30-mesh sieve to sieve and blend low-substituted hydroxypropylcellulose (9g), magnesium stearate (1.3g) and silicon dioxide (0.6g). Then the obtained granules are pressed into a plain tablet with a hardness of 3-10 kg using a punching film with a diameter of 6.5 mm.
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com