Escherichia coli fusion expression plectasin, preparation method and application thereof
A Plectasin, fusion expression technology, applied in the application, chemical instruments and methods, expression enhancement stability/folded protein fusion, etc., can solve the problem of high cost, unsuitable for antibacterial drug preparations, health products or preservatives, impact Problems such as the expression of plectasin antimicrobial peptides, to achieve soluble expression and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
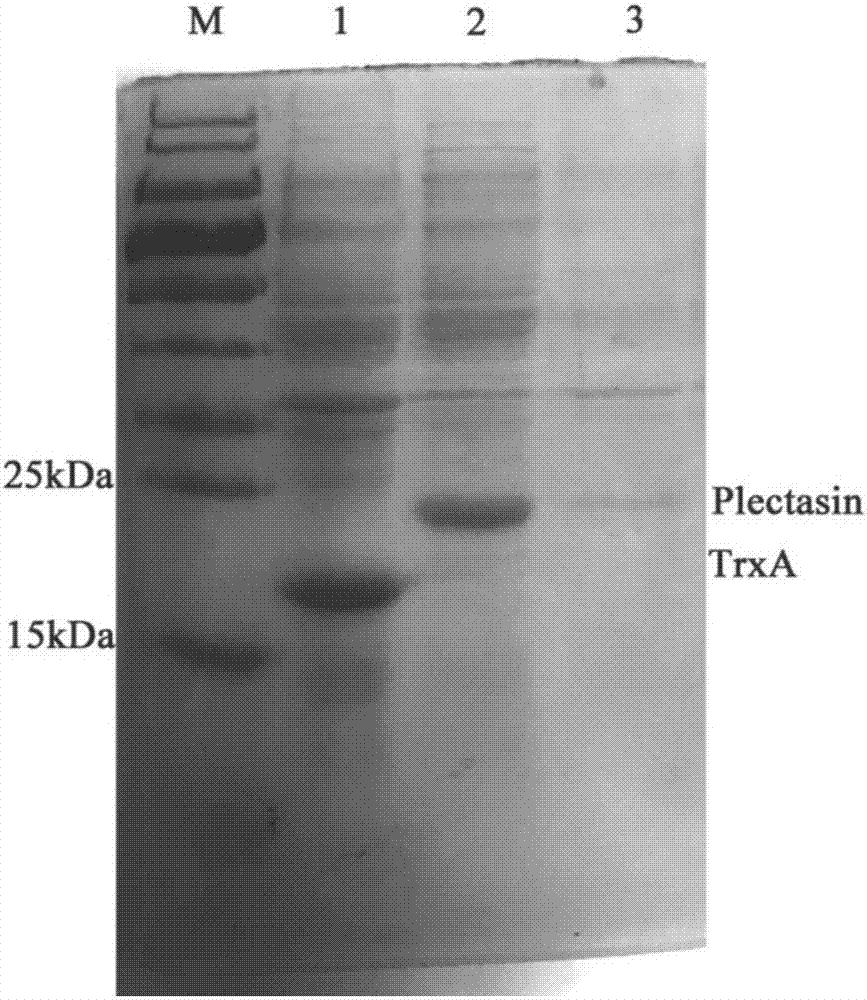
[0021] The preparation process of embodiment 1 plectasin fusion protein
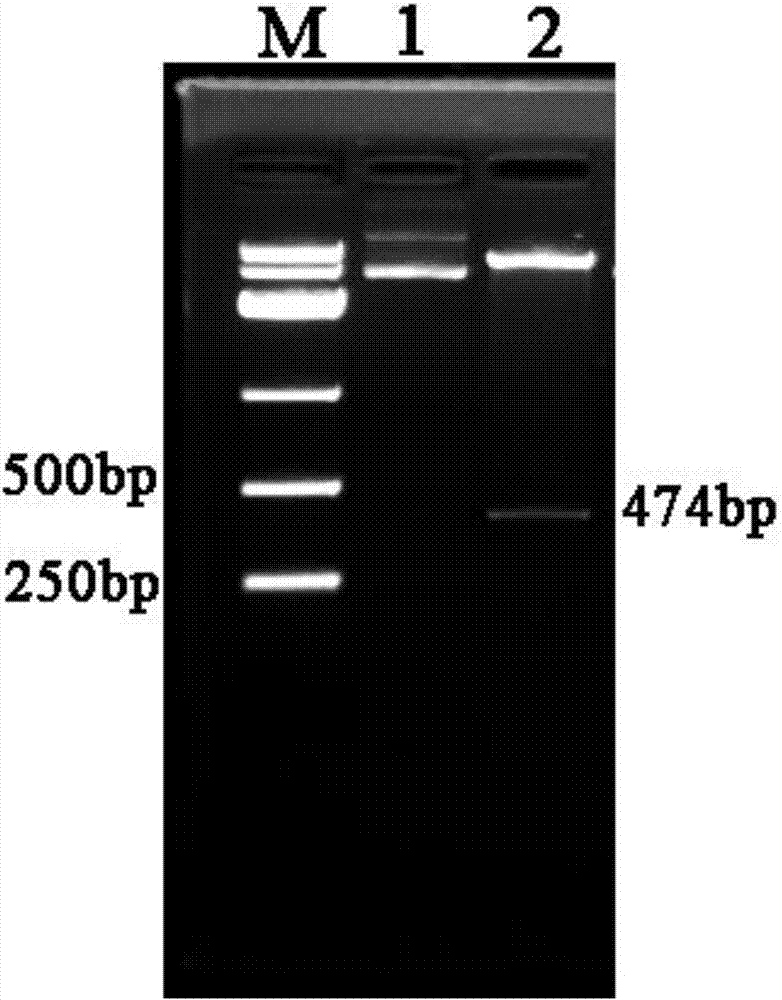
[0022] (1) Design and synthesis of Plectasin fusion protein gene
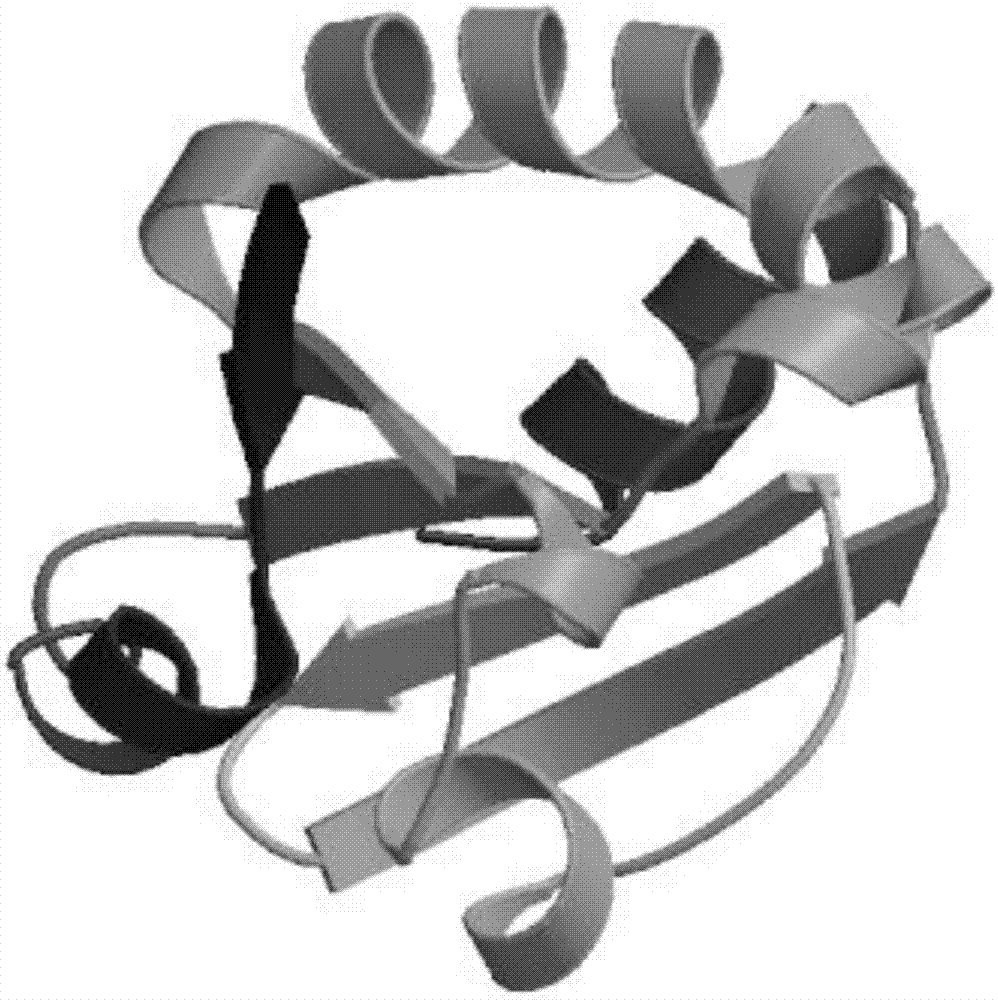
[0023] According to the amino acid sequence of Ascomyces saprophyticum Plectasin reported in the published literature (Mygind PH, et al. Nature, 2005; 437(13):975-980) and the amino acid of thioredoxin (Trx) in Genbank Sequence, then refer to the preferred codons of Escherichia coli to design the plectasin fusion protein gene, and use the GGGGS flexible Linker to connect the plectasin and Trx. The designed gene sequence is as follows:
[0024] CATATG AGCGATAAAATTATTCACCTGACTGACGACAGTTTTGACACGGATGTACTCAAAGCGGACGGGGCGATCCTCGTCGATTTCTGGGCAGAGTGGTGCGGTCCGTGCAAAATGATCGCCCCGATTCTGGATGAAATCGCTGACGAATATCAGGGCAAACTGACCGTTGCAAAACTGAACATCGATCAAAACCCTGGCACTGCGCCGAAATATGGCATCCGTGGTATCCCGACTCTGCTGCTGTTCAAAAACGGTGAAGTGGCGGCAACCAAAGTGGGTGCACTGTCTAAAGGTCAGTTGAAAGAGTTCCTCGACGCTAACCTGGCC GGTGGCGGTGGTAGTATGGGCTTTGGCTGTAATGGTCCGTGGGATGAAGATGATA TGCAGTGC...
Embodiment 2
[0031] Antibacterial and bactericidal experiments of embodiment 2 Plectasin fusion protein
[0032] (1) Disk method to detect the antibacterial effect of plectasin fusion protein
[0033] The test strains were clinical methicillin-resistant Staphylococcus aureus MRSA15471114, MRSA15471118 and penicillin-resistant Streptococcus pneumoniae PRSP31355, among which Staphylococcus aureus MRSA15471114 and MRSA15471118 were cultured in M-H medium, and penicillin-resistant Streptococcus pneumoniae PRSP31355 was cultured with 5% Culture sheep blood in M-H medium, cultivate at 37°C until OD600=0.5, take 100 μL of bacterial solution and spread evenly on the agar plate. Place the autoclaved paper evenly on the surface of the agar plate, and add 30 μL of 1 mg / mL plectasin and 10 μL of 1 mg / mL cephalexin dropwise on the paper. Add 30 μL ddH dropwise 2The disk of O was used as a negative control. The results showed that plectasin fusion protein had antibacterial effect on three clinical dr...
Embodiment 3
[0036] Example 3 Acute Toxicity Test of Plectasin Fusion Protein in Mice
[0037] The purpose of this experiment is to observe whether plectasin fusion protein has toxic effects on mice. Forty healthy BALB / c mice, half male and half male, weighing 22±0.31 g, were used. Plectasin fusion protein 1mg / mL, intramuscularly injected 1 time a day, 0.1mL / time, continuously injected for 7 days, and the toxic reaction in mice was observed. The experimental results showed that during the experiment, the mice had no abnormal reaction, their diet and activities were normal, and all 40 mice survived. The mice were killed, and no abnormalities were found in the heart, liver, lung, spleen, kidney, gastrointestinal tract and other organs. It is proved that the plectasin fusion protein has no toxic effect on animals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com