Alginate lyase and preparation method and application thereof
A technology of alginate lyase and brown algae lyase, applied in the directions of lyase, carbon-oxygen lyase, biochemical equipment and methods, etc., can solve the problems of narrow enzyme substrate spectrum, poor enzyme stability, low enzyme activity, etc. The properties are stable, the product is uniform, and the effect of improving the enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Purification of brown algae lyase in the original bacteria
[0070] The purification of brown algae lyase in the original bacteria comprises the following steps:
[0071] (1) Preparation of crude enzyme solution:
[0072] (a) Take Bacillus Alg07 stored at -80°C, streak on the optimized solid medium, and culture in a 30°C incubator;
[0073] (b) Pick a single colony, inoculate it into a 30 mL test tube containing 5 mL of fermentation medium, and incubate with shaking at 30°C for 16 hours;
[0074] (c) Inoculate into a 250mL Erlenmeyer flask containing 40mL of fermentation medium with an inoculum size of 0.5% (v / v), and cultivate for 24h at 30°C and 180r / min;
[0075] (d) Collect the bacteria cultured in step (c), centrifuge at 4°C and 12000r / min for 30min, take the supernatant, discard the bacteria precipitate, and obtain the crude enzyme solution.
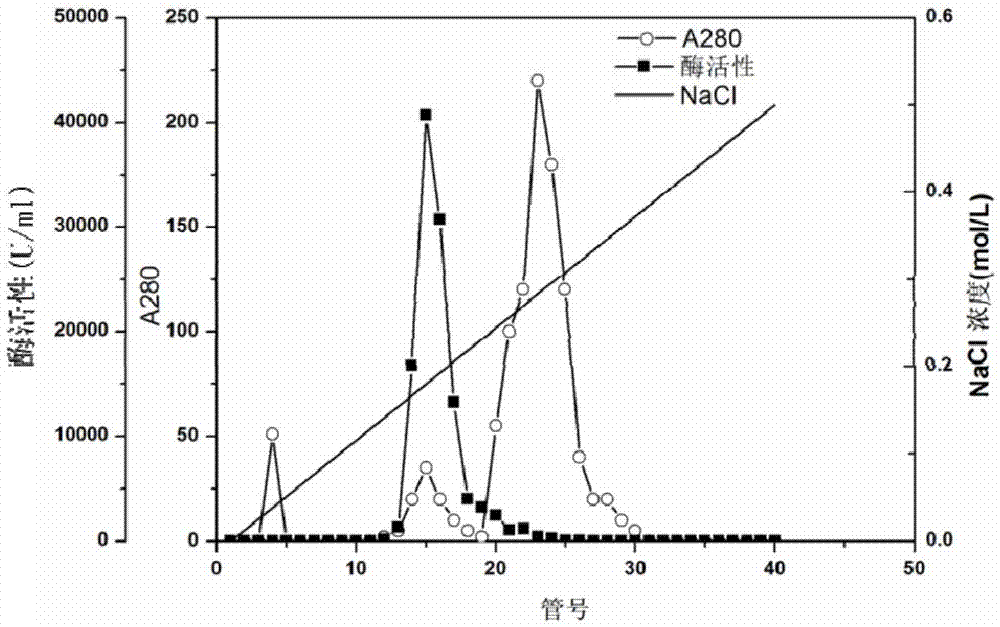
[0076] (2) Concentrate the crude enzyme solution obtained in step (1): take 1000mL of fermentation supernatan...
Embodiment 2
[0083] Example 2 N-terminal sequencing of brown algae lyase
[0084] (1) Establish standard amino acid map
[0085] ① Using the mixed amino acid standard (PTH-AA), run under normal conditions to generate a standard chromatogram
[0086] ② Correct the retention time of the mixed amino acid standard and generate a standard method file
[0087] (2) On-board inspection
[0088] The PVDF membrane is sandwiched between two PTFE filter membranes, placed in the reactor of the protein sequencer PPSQ-31A, and the number of cycles is set.
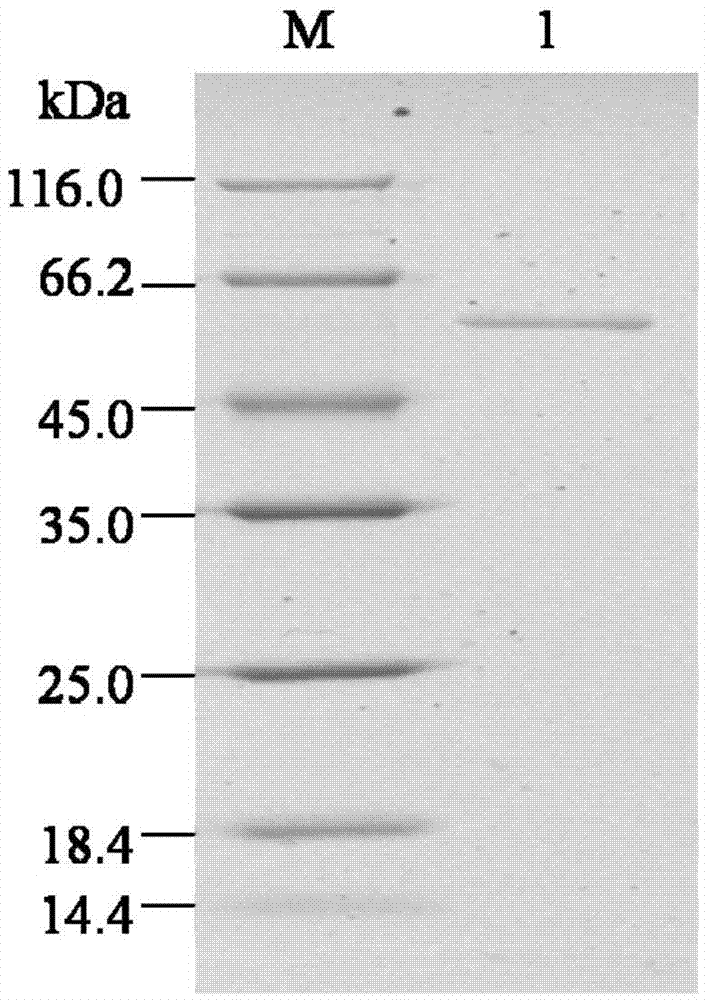
[0089] (3) Transfer the brown algae lyase SEQ ID NO.2 to PVDF membrane.
Embodiment 3
[0090] Example 3 Gene mining of alginate lyase
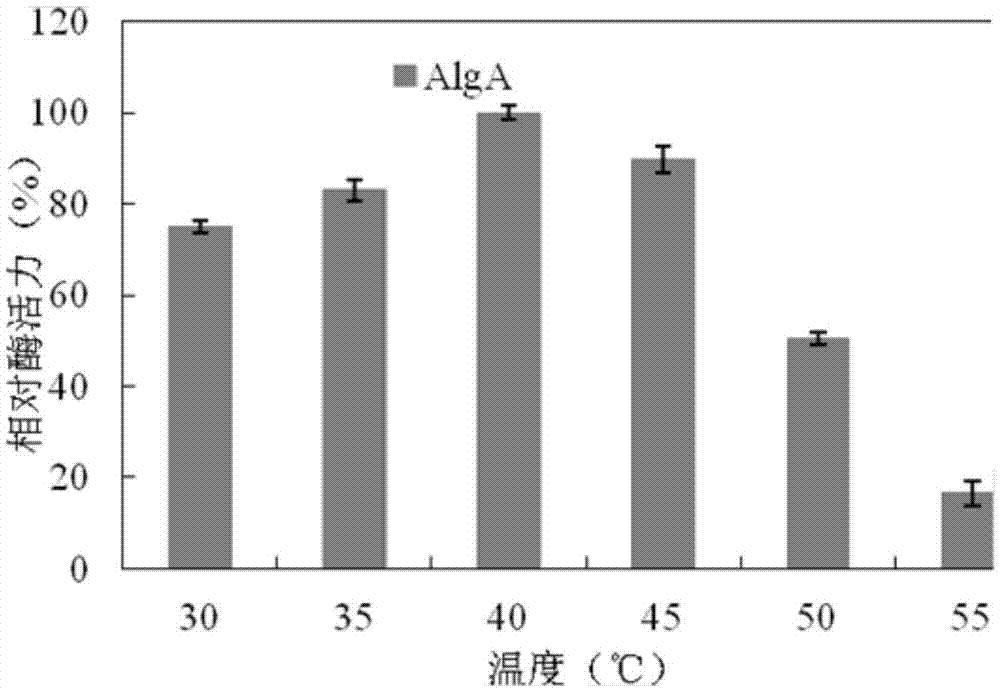
[0091] The results analyzed by the RAST software showed that the genome DNA of the Bacillus weihaiensis Alg07 strain carried the coding gene No.2004 of alginate lyase. Analyzed by the biological software DNAMAN, it was shown that the theoretical molecular weight of the protein (AlgA) encoded by gene No.2004 was about 143kD. The signal peptide online prediction software SignalP4.1Server was used to analyze, and the result showed that the amino acid sequence of protein 2004 contained secreted signal peptide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com