Preparation method and device of aromatic chloride
A chloride and aromatic technology, which is applied in the field of devices for realizing the above-mentioned aromatic chloride preparation method, can solve the problems of shrinkage, high safety risk, poor heat transfer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
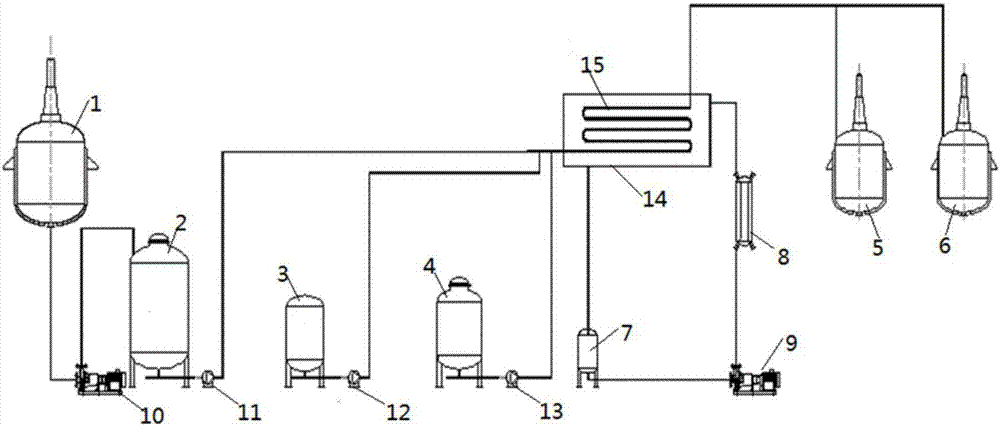
[0089] This tubular reaction device, such as figure 1 As shown, it includes: raw material storage tanks 2, 3, 4, crystallization tanks 5, 6, feed pumps 11, 12, 13, tubular reactor 15, liquid storage tank 7, heat exchanger 8, circulation pump 9 , a heat exchange liquid tank 14, a raw material dissolution kettle 1, and a transfer pump 10.
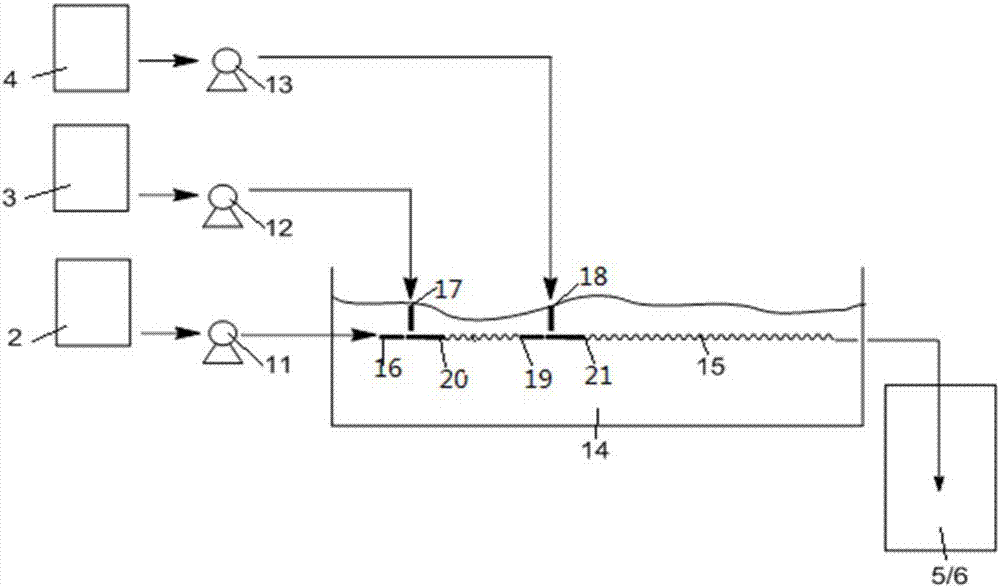
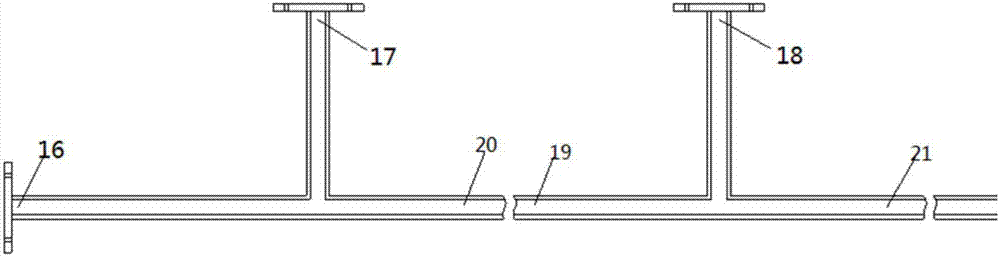
[0090] The number of raw material storage tanks, feed pumps and corresponding material inlets can be set as required. Such as figure 2 As shown, when there are two materials in the reaction that are first added to the tubular reactor, and then react with another added material in the tubular reactor 15, ports 16, 17, 20 and ports 18, 19, 21 They are the ports of two three-way hollow pipes respectively; port 21 is connected with tubular reactor 15; ports 16, 17 and 18 are material inlets of tubular reactor 15; material inlets 16, 17 and 18 are respectively connected with feed pump 11 , 12 and 13 are hermetically connected; the feed pumps 1...
Embodiment 2
[0098]
[0099] 1. Raw material preparation: Compound A-1 and acetic acid were stirred at 60°C for 30 minutes to dissolve and be configured into a solution (mass fraction 35.7%) placed in raw material tank 2, hydrogen peroxide (mass fraction 50%) placed in raw material tank 3, hydrochloric acid (mass fraction Fraction 36%) is placed in raw material tank 4.
[0100] 2. Utilize the tubular reactor of embodiment 1, reactor pipe inner diameter 4mm and pipe length 40m, according to the following steps: (1) by metering pump control, the flow rate of compound A-1 solution is 76ml / min, the flow rate of hydrogen peroxide is 10ml / min mixed first, residence time 2s; (2) mixed with hydrochloric acid controlled by a metering pump at 28ml / min, and reacted at an external temperature of 70°C, residence time 4.3min; (3) adding the effluent of the tubular reactor into water, Adjust to non-oxidizing with sodium sulfite, filter, wash the filter cake with water, and dry to obtain product B-1. ...
Embodiment 3
[0103] 1. Raw material preparation: Compound A-1 and acetic acid were stirred at 60°C for 30 minutes to dissolve and be configured into a solution (35.7% by mass fraction) placed in raw material tank 2, hydrogen peroxide (35% by mass fraction) placed in raw material tank 3, hydrochloric acid (mass fraction 35%) Fraction 36%) is placed in raw material tank 4.
[0104] 2. Utilize the tubular reactor of embodiment 1, reactor pipe internal diameter 4mm and pipe length 32m, according to the following steps: (1) by metering pump control, the flow rate of compound A-1 solution 51ml / min, the flow rate of hydrogen peroxide 9.7 ml / min first mixed, residence time 2s; (2) mixed with hydrochloric acid controlled by a metering pump at 19ml / min, and reacted at an external temperature of 70°C, residence time 5min; (3) adding the effluent from the tubular reactor into water, Adjust to non-oxidizing with sodium sulfite, filter, wash the filter cake with water, and dry to obtain product B-1.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com