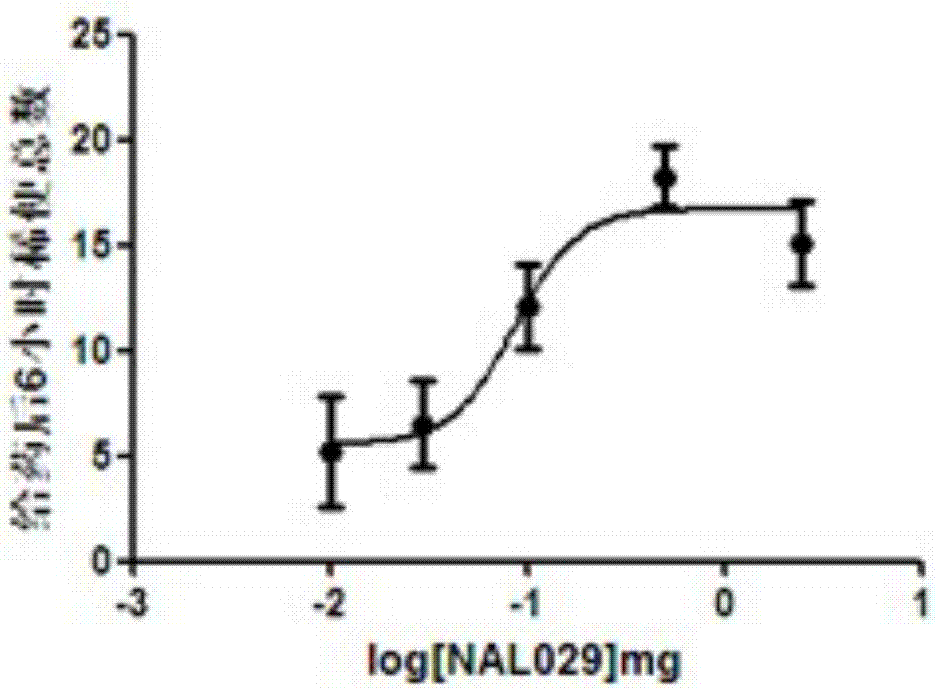
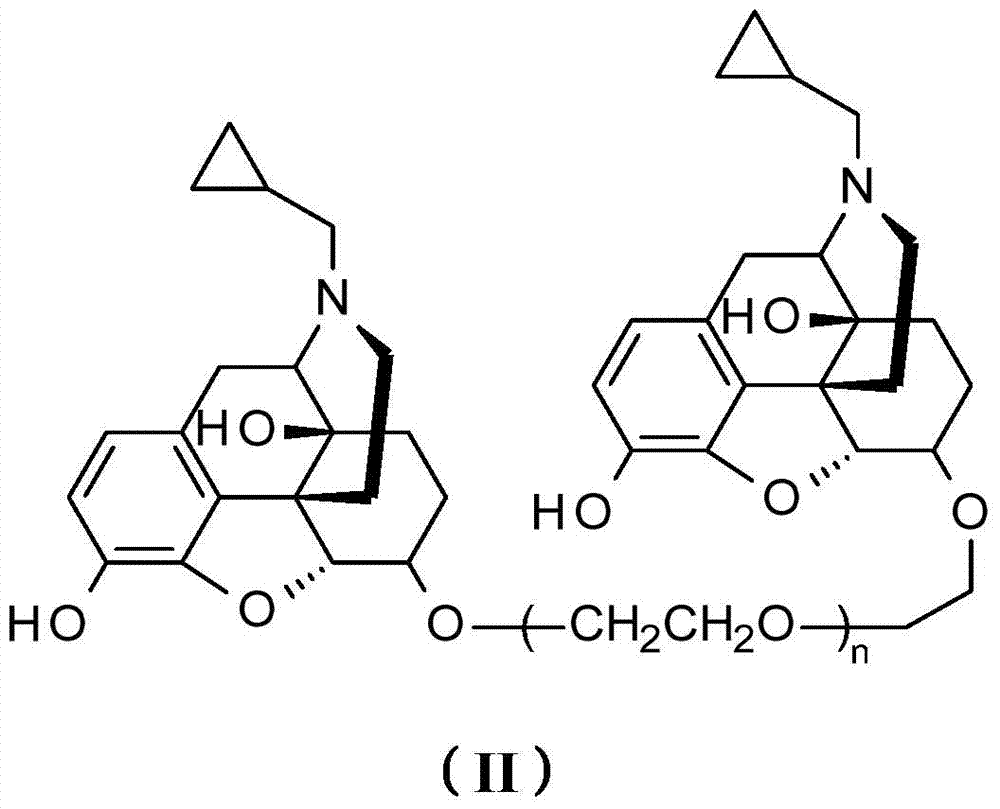
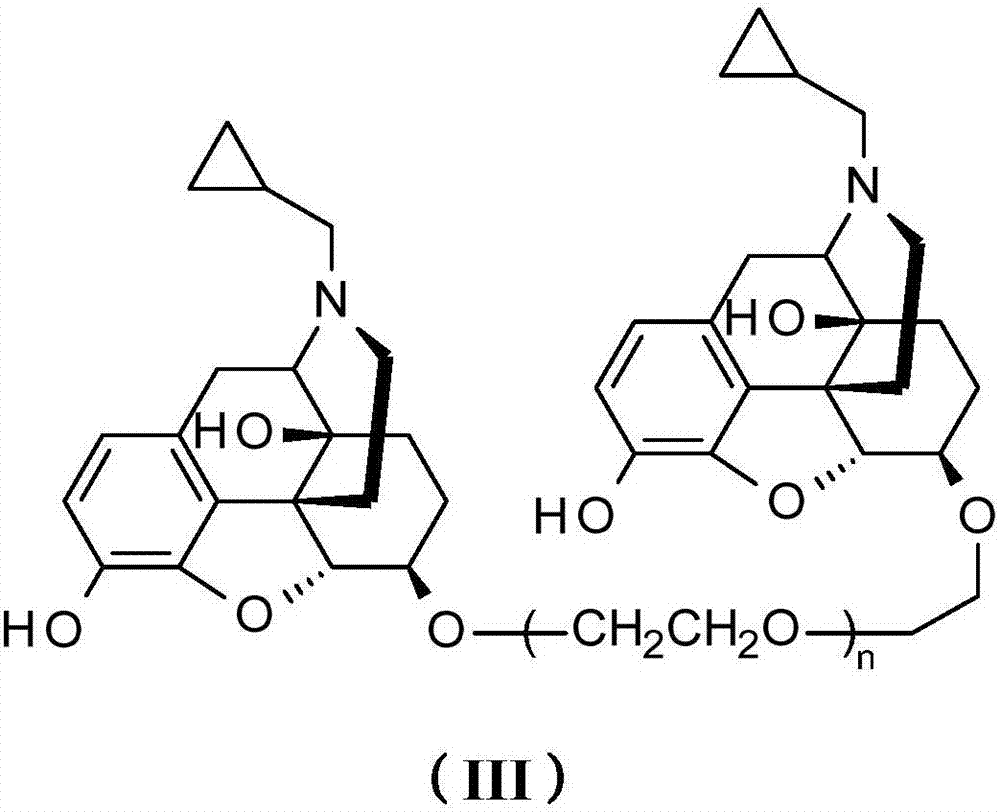
Opiate receptor antagonist conjugates and application thereof
A technology of receptor antagonists and conjugates, applied in the field of conjugates covalently coupled with hydrophilic polymers and opioid receptor antagonists, can solve diarrhea and cramps, unpredictable effects, laxatives non-specific issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1: Preparation of polyethylene glycol mesylate
[0088]
[0089] Add 50ml dichloromethane, 7.18ml triethylamine, 206mg p-dimethylaminopyridine and 10.0g CH in the reaction flask 3 -(OCH 2 CH 2 ) 6 -OH (abbreviated as mPEG6-OH) (Jiaxing Bomei, M006110503). Add 3.28ml methanesulfonyl chloride in 50ml dichloromethane solution dropwise with stirring at 0°C. After the addition, the reaction was stirred overnight at room temperature. The reaction solution was transferred to a separatory funnel and washed with dilute hydrochloric acid and brine successively. The organic layer was separated, dried with 30 g of anhydrous sodium sulfate, filtered to remove the desiccant, and concentrated by rotary evaporation to obtain 13.6 g of crude product. The crude product was purified by chromatography to obtain 13.0g mPEG6-OMs(CH 3 -(OCH 2 CH 2 ) 6 -OSO 2 CH 3 ). 1H-NMR (400MHz, CDCl3), δppm 4.35-4.41 (2H, m), 3.74-3.80 (2H, m), 3.60-3.70 (18H, m), 3.52-3.58 (2H, m), 3.38 (3H) , S...
Embodiment 2
[0100] Example 2: Preparation of 3-MOM-naltrexone and 3-MOM-naloxone
[0101]
[0102] 200ml of dichloromethane, 41.7g of diisopropylethylamine and 19.83g of naltrexone hydrochloride (Jinan Haohua Industrial Co., Ltd., FR00007) were sequentially added to the reaction flask. 17.7 g of chloromethyl methyl ether in 200 ml of dichloromethane was added dropwise with stirring at 0°C. After the addition was completed, the reaction was stirred overnight. The reaction solution was transferred into a separatory funnel and washed with lye (400ml water+40g anhydrous sodium carbonate+10.0g sodium hydroxide+30g sodium chloride and mixed). The organic layer was separated, dried with 30 g of anhydrous sodium sulfate, filtered to remove the desiccant, and concentrated by rotary evaporation to obtain 21.3 g of product 3-MOM-naltrexone. 1H-NMR (400MHz, CDCl3), δppm 6.85-6.92 (1H, d, J=8.0Hz), 6.58-6.65 (1H, d, J=8.4Hz), 5.20-5.30 (2H, m), 4.68 (1H , S), 3.52 (3H, s), 3.15-3.22 (1H, d, J=6.0 Hz),...
Embodiment 3
[0104] Example 3: Preparation of 3-MOM-6-α-naltrexol and 3-MOM-6-α-naloxol
[0105]
[0106] Under the protection of argon, 100ml of tetrahydrofuran and 16.8g of 3-MOM-naltrexone were added to the reaction flask. While stirring at -10°C, add 45ml of a tetrahydrofuran solution of potassium tri-sec-butylborohydride. Use thin layer chromatography (TLC) to check the progress of the reaction. After the reaction is completed, adjust the pH to 2-4 with dilute hydrochloric acid. The reaction solution was transferred to a separatory funnel, and extracted with dichloromethane 4 times (150, 100, 100, 100 ml). Separate the organic layer, add 10.0 g of sodium hydroxide in 150 ml of aqueous solution to the water layer, mix well, and extract 3 times with dichloromethane (100 ml×3). The organic layers were combined, dried with 20 g of anhydrous sodium sulfate, filtered to remove the desiccant, and concentrated by rotary evaporation to obtain 16.5 g of the product 3-MOM-6-α-naltrexol. 1H-NMR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com