Preparation method and application of polyclonal antibody against chicken caspase-1
A technology for polyclonal antibodies and uses, applied in the fields of botanical equipment and methods, biochemical equipment and methods, antibodies, etc., can solve problems such as restricting research, and achieve the effect of simple separation and purification method, low cost and short cycle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Construction of pET-32a(+)-caspase-1 prokaryotic expression plasmid
[0023] 1. Design and synthesis of primers
[0024] According to the amino acid sequence of chicken caspase-1 mRNA (accession number: AF031351.1) in GenBank, and referring to the multiple cloning site sequence of the prokaryotic expression vector pET-32a(+), a pair of primers (underlined as enzyme cleavage sites) were designed using Oligo7 software point):
[0025] Forward-Primer: 5'- GAATTC ATGAGCAGGGCAAGATCTTC-3'
[0026] Reverse-Primer: 5'- CTCGAG GAGGCCTGGGAAGAGAT-3'
[0027] Forward-Primer and Reverse-Primer are upstream and downstream primers respectively, and EcoR I (GAATTC) and Xho I (CTCGAG) restriction sites are added to the 5' end respectively.
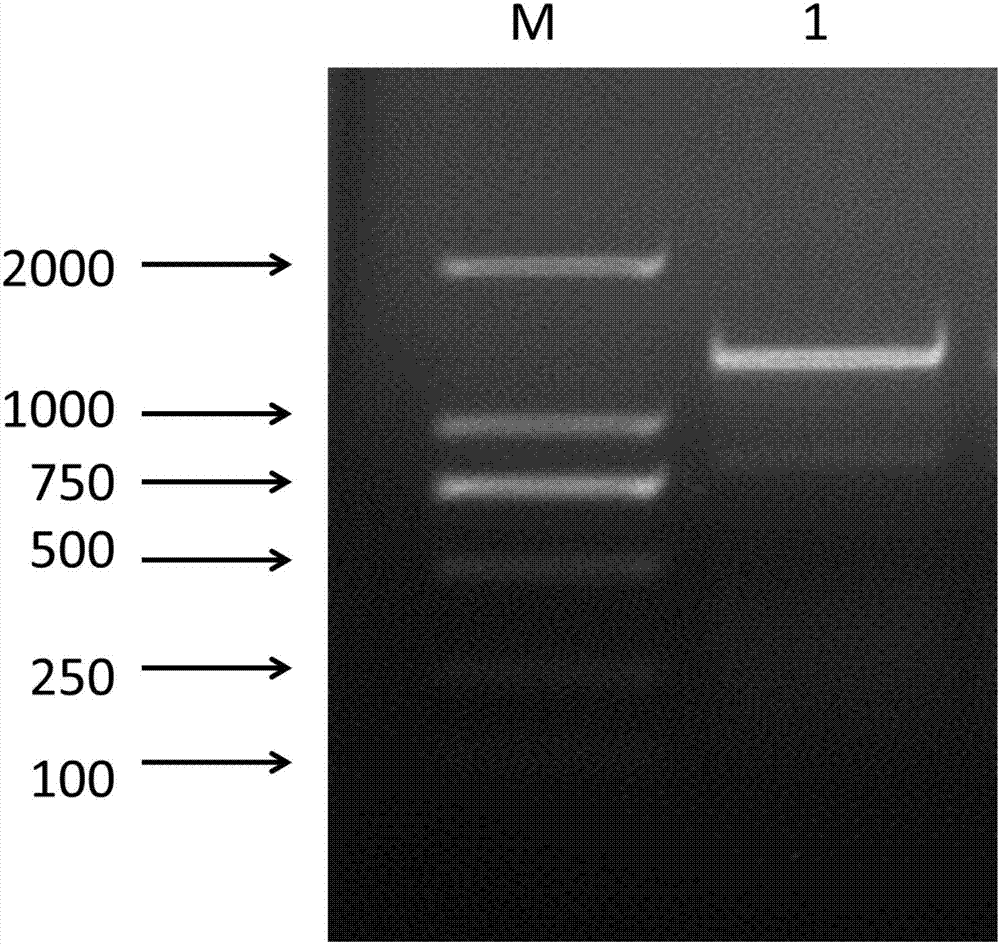
[0028] 2. Amplification of chicken caspase-1 gene
[0029] Extract total RNA from chicken lung tissue, and reverse it into cDNA; use the cDNA as a template, and use the above-mentioned primers to perform PCR amplification to ob...
Embodiment 2
[0034] Example 2: Induced expression and identification of expression products
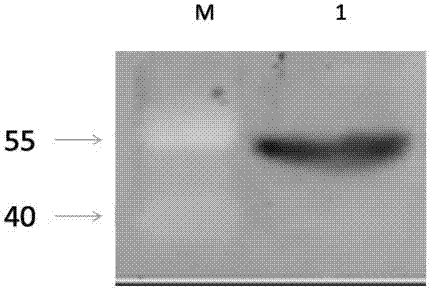
[0035] Transform the recombinant prokaryotic expression plasmid into BL21 host bacteria, pick the positive monoclonal bacteria and inoculate them into the LB (Amp+) medium for culture, shake the culture at 200rpm / min, when the OD600 is about 0.6, add IPTG at the final concentration of 1mM, 37℃ Induce the expression for 12 hours, collect the bacteria after centrifugation, add 4°C pre-cooled PBS to the pellet and resuspend, after ultrasonic cracking, separate the supernatant and the pellet, fully dissolve the pellet with a phosphate solution containing 8M urea, and remove it after centrifugation at 4°C Precipitate and keep the supernatant. Add 5×SDS loading buffer to the supernatant, mix well, and then bathe in boiling water for 10 minutes to obtain unpurified protein samples. SDS-PAGE and western blot methods are used to identify protein expression, such as image 3 shown.
Embodiment 3
[0036] Embodiment 3: Purification of recombinant protein
[0037] Prepare several pieces of 10% 1.5mm SDS-PAGE protein glue, run the above unpurified protein samples on SDS-PAGE gel, stain with 0.25mol / L pre-cooled KCL after running the gel, shake on the shaker for 5min, you can see When an obvious and specific white band appears near the size of the target band, after it is determined to be the target band, carefully cut it off with a scalpel and move it to a clean plate, wash it with pre-cooled PBS for 3 times, and then Put the gel strip into a clean mortar, grind it with liquid nitrogen, add 500uL PBS, transfer to a clean EP tube, stay at 4°C overnight, centrifuge at 4°C to take the supernatant, which is the purified target protein . SDS-PAGE and western blot methods can be used to identify the purification of the target protein, such as Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com