Photochromic compound and preparation method and application thereof
A photochromic and compound technology, applied in chemical instruments and methods, color-changing fluorescent materials, luminescent materials, etc., can solve the problems of reducing the effect of photochromic fluorescent switches, achieve high yield, simple preparation method, and easy industrialization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
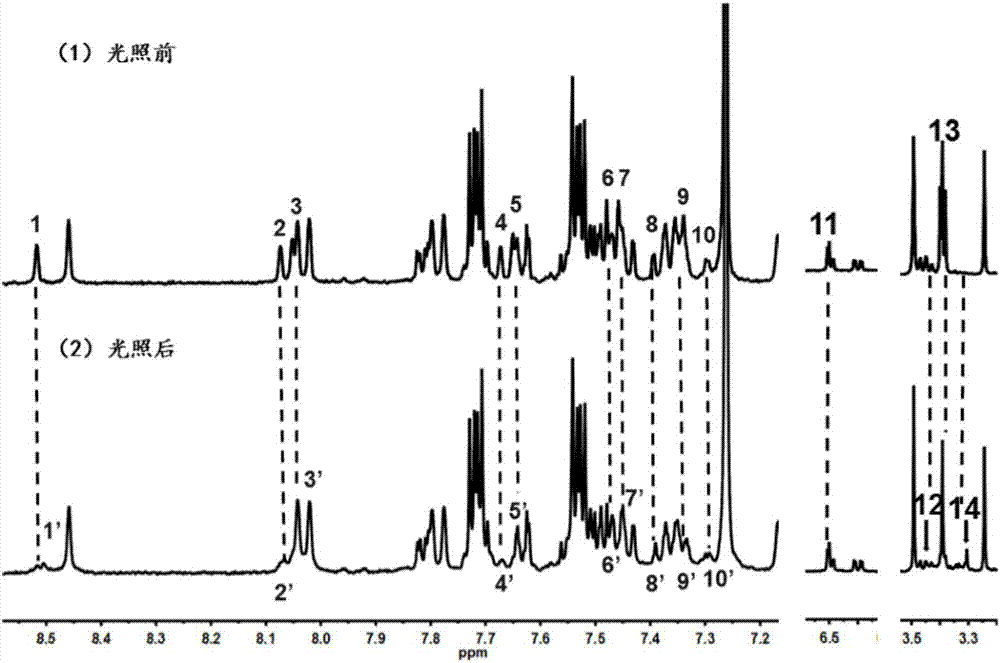
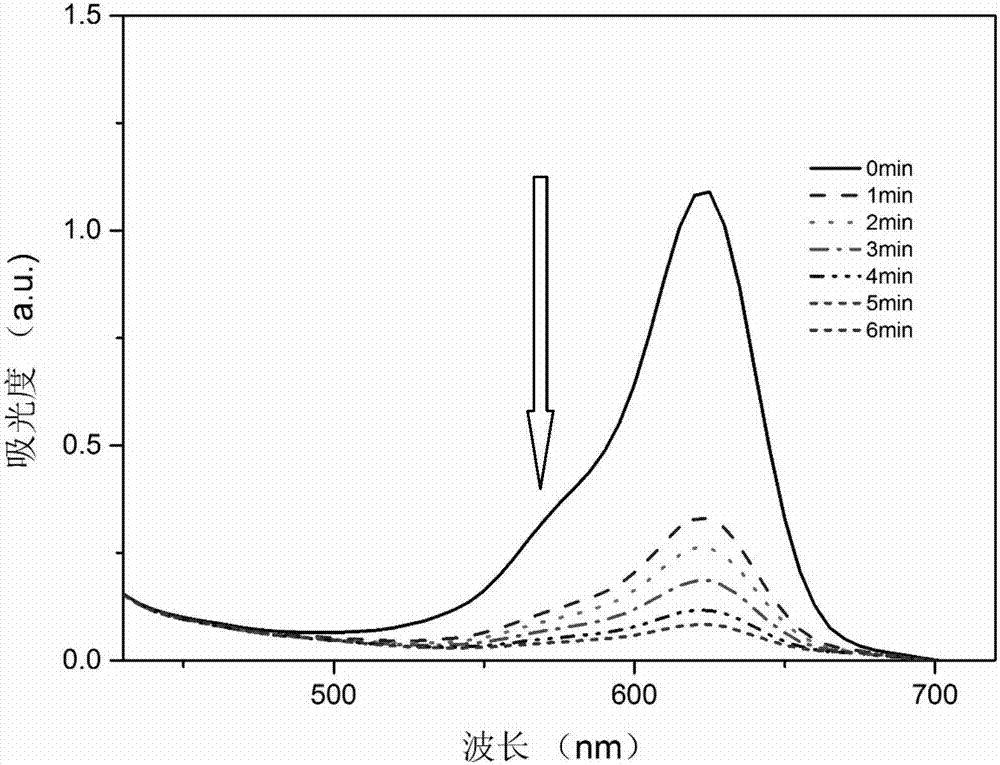
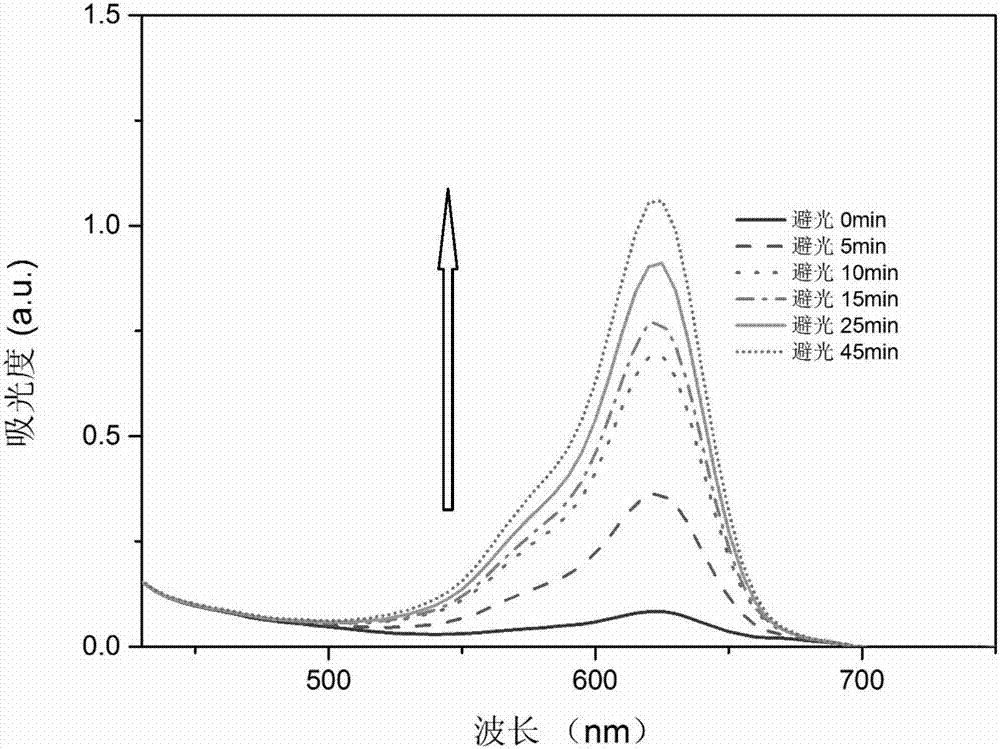
Image
Examples
Embodiment 1
[0058] The synthesis of embodiment 1 compound A1
[0059] synthetic route
[0060]
[0061] (a): Synthesis of compound 2 (pinacol 9-anthracene borate)
[0062] Take 9-bromoanthracene (1.7g, 6.6mmol), and diboronic acid pinacol ester (2g, 7.9mmol) were dissolved in 1,4-dioxane (20mL), and anhydrous potassium acetate (1.9g, 19.8 mmol) and [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (0.6g, 0.7mmol), were added to the above system, heated to reflux at 100°C for 2h, TLC (thin layer chromatography ) Follow up and monitor the reaction until complete. Suction filtration, rotary evaporation to remove 1,4-dioxane, washing with saturated brine, extraction with dichloromethane three times, the combined organic phases were dried with anhydrous sodium sulfate, spin-dried, and separated by column chromatography (eluent was petroleum Ether: ethyl acetate = 100:1), to obtain a light yellow powder, that is, compound 2 (1.8 g, yield 90%).
[0063] (b): Synthesis of compound...
Embodiment 2
[0076] The synthesis of embodiment 2 compound A2
[0077] synthetic route
[0078]
[0079] (a): Synthesis of compound 7 (N-n-butyl-1,8-naphthoimide boronic acid pinacol ester)
[0080] Take 4-bromo-N-ethyl-1,8-naphthalimide (2.2g, 6.6mmol), diboronic acid pinacol ester (2g, 7.9mmol) was dissolved in 1,4-dioxane (20mL ), anhydrous potassium acetate (1.9g, 19.8mmol) and [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (0.6g, 0.7mmol) were added to the above system , heated to reflux at 100° C. for 2 h, followed by TLC (thin layer chromatography) to track and monitor the reaction until it was complete. Suction filtration, rotary evaporation to remove 1,4-dioxane, washing with saturated brine, extraction with dichloromethane three times, the combined organic phases were dried with anhydrous sodium sulfate, spin-dried, and separated by column chromatography (eluent was petroleum Ether:ethyl acetate=100:1) to obtain a light yellow powder, that is, compound 7 (2.1 g,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com