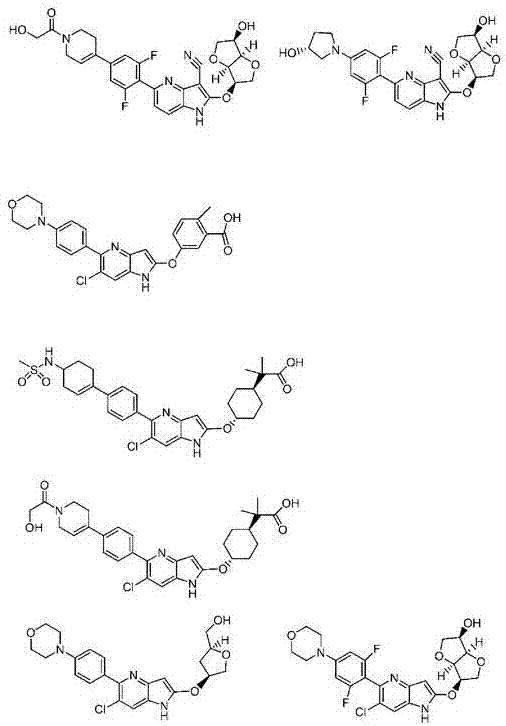
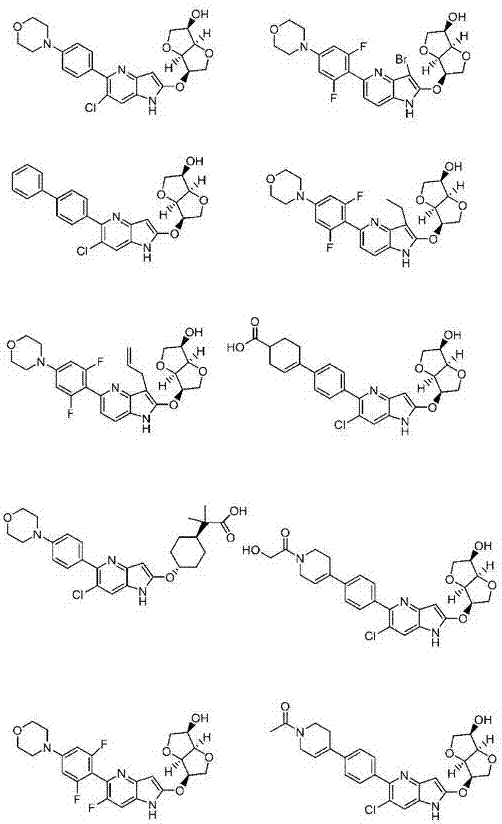
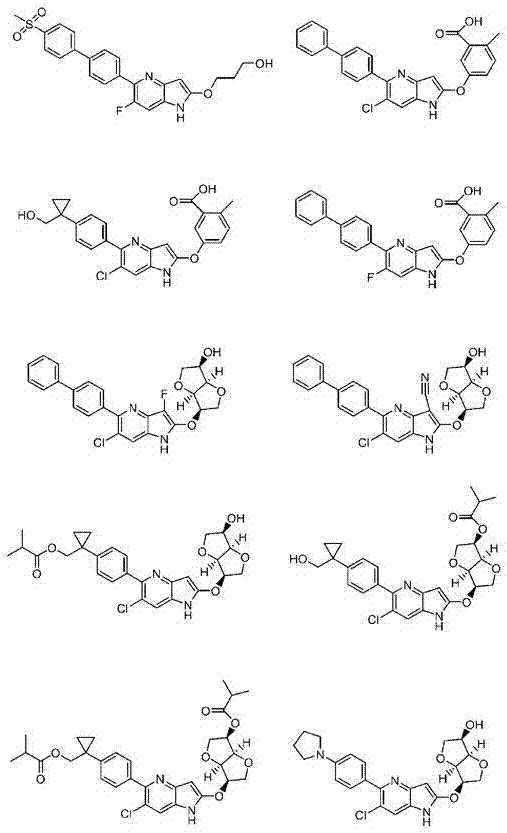
Azaindole derivative having AMPK-activating effect
A kind of compound, the technology of heterocyclic group is applied in the field of azaindole derivatives with AMPK activation effect, and can solve the problems such as no disclosure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0758] [chemical formula 74]
[0759]
[0760] After compound 1 (1.70 g, 8.81 mmol) was dissolved in DMF (17 mL), tert-butylethyl malonate (3.34 ml, 17.62 mmol) was added and cooled with an ice bath. 60% NaH (705 mg, 17.62 mmol) was added thereto, and stirred at room temperature. After the reaction was completed, the reaction solution was cooled in an ice bath, and then 2 mol / L aqueous hydrochloric acid (10 ml) was added, followed by extraction with ethyl acetate. Thereafter, the organic layer was washed with water. The obtained organic layer was dried over magnesium sulfate, and then concentrated under reduced pressure to distill off the solvent. The resulting residue was purified by silica gel column chromatography to obtain Compound 2 (2.69 g, 88.8%).
[0761] Compound 2: 1H-NMR (CDCl 3 )δ: 1.30 (3H, t, J = 7.2 Hz), 1.49 (9H, s), 4.25-4.36 (2H, m), 5.38 (1H, s), 8.46 (1H, s), 8.77 (1H, s ).
[0762] Compound 2 (1.00 g, 2.90 mmol) was dissolved in chloroform (5 mL) ...
Embodiment 2
[0767] [chemical formula 75]
[0768]
[0769] Dilute 2,6-difluorophenylmethanol (14.202g, 99mmol) in DMF (50ml), add 60%Wt NaH (3.58g, 90mmol) under ice-water cooling, and stir at 0°C for 3 minutes Then, stir at room temperature. Compound 4 (5000 mg, 17.92 mmol) dissolved in DMF (10 ml) was added to the reaction solution, followed by further stirring at room temperature. The reaction liquid was cooled, 2 mol / L hydrochloric acid aqueous solution was added, and ethyl acetate was used for extraction. The organic layer was washed with water and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography to obtain Compound 5 (4.9053 g, 70.8%).
[0770] Compound 5: Method B
[0771] LC / MS retention time = 2.49 min.
[0772] MS (ESI) m / z = 386.95 (M+H)+.
[0773] Compound 5 (4800 mg, 12.41 mmol) was dissolved in THF (25 ml) and methanol (25 ml), and ammonium chloride (3319 mg, 62.1 mmol) dissolved in water (12.5 ml) was added....
Embodiment 3
[0802] [chemical formula 76]
[0803]
[0804] Compound 13 was synthesized from compound 4 by the same method as compound 6.
[0805] Compound 13: Method B
[0806] LC / MS retention time = 1.21 min.
[0807] MS (ESI) m / z = 202.85 (M+H)+.
[0808] Compound 14 was synthesized from compound 13 by the same method as compound 7.
[0809] Compound 14: Method B
[0810] LC / MS retention time = 2.98 min.
[0811] MS (ESI) m / z = 352.65 (M+H)+.
[0812] Compound 14 (325 mg, 0.924 mmol) and isomannitol (1350 mg, 9.24 mmol) were added to DMF (3.0 ml), 60% Wt NaH (111 mg, 2.77 mmol) was added, and stirred at room temperature for 5 minutes. Thereafter, heating and stirring were performed at 120°C. After returning the reaction liquid to room temperature, ethyl acetate was added, and washed with 1 mol / L aqueous hydrochloric acid and water. The obtained organic layer was dried over magnesium sulfate, and then concentrated under reduced pressure to distill off the solvent. The resulti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com