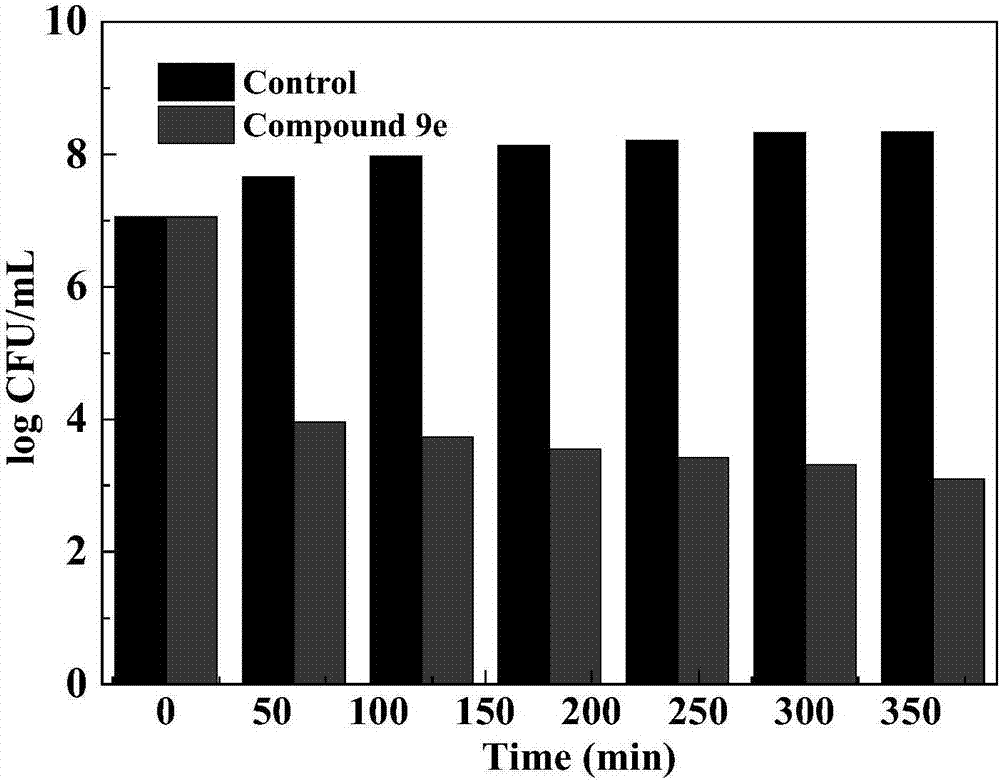
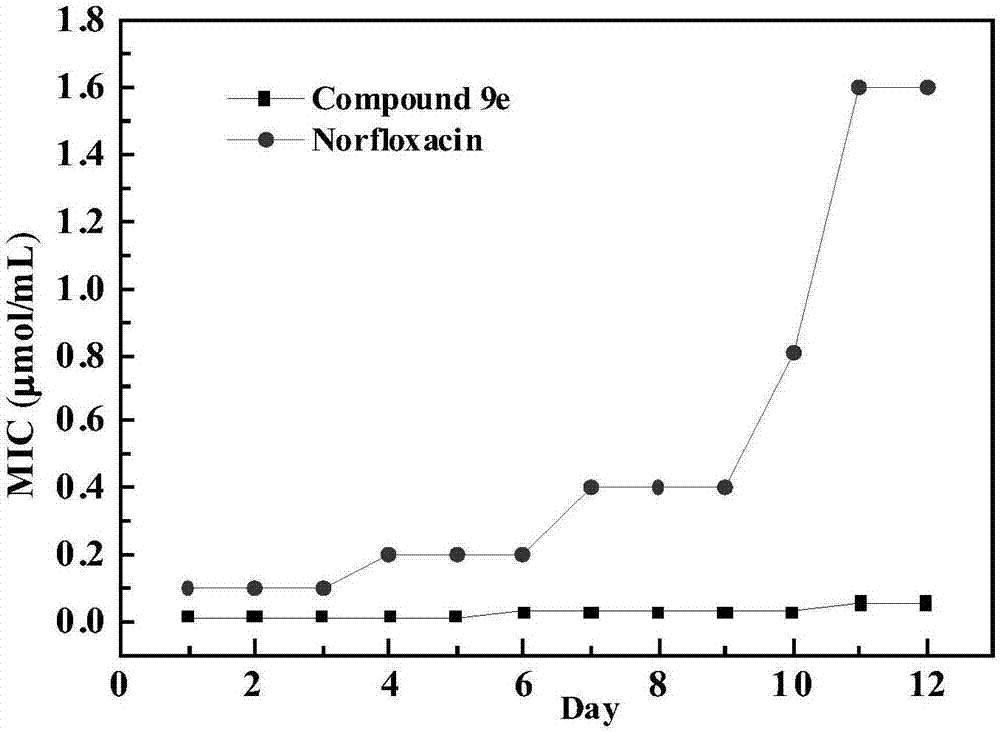
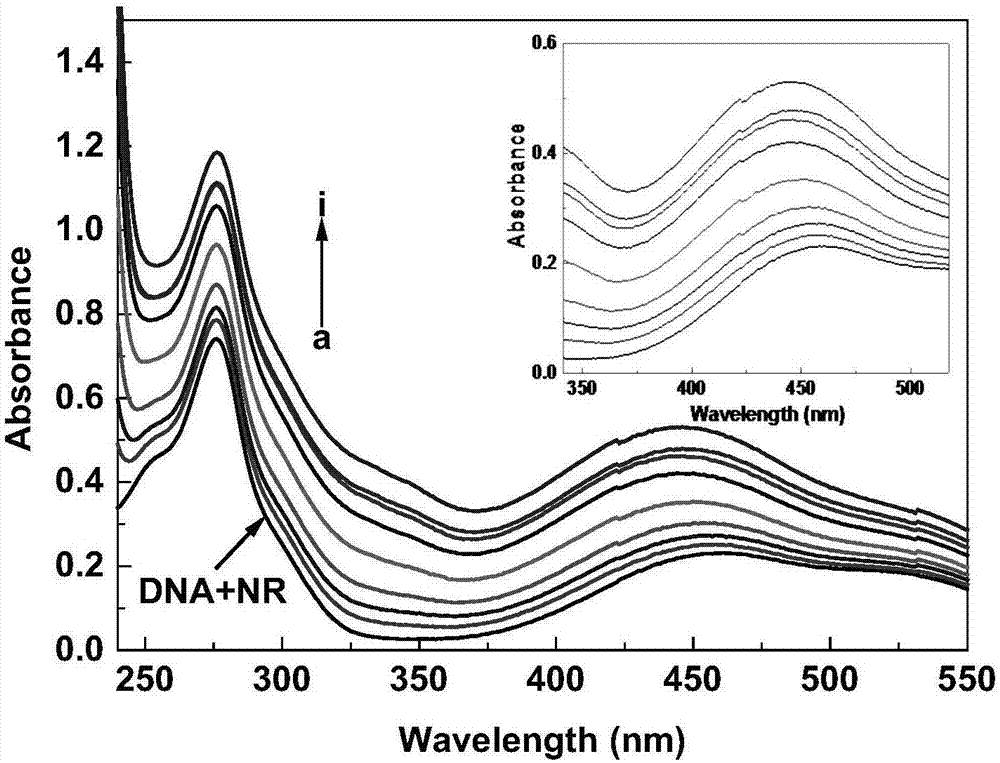
Alicyclic amine type naphthalimide metronidazole derivative and preparation method and application thereof
A technology of naphthylimide metronidazole and alicyclic amine is applied in the field of chemistry and achieves the effects of easy availability of raw materials, simple preparation method and fast sterilization speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1, the preparation of intermediate III
[0036]
[0037] The method described in the reference "Luo Y L, Kishore B, Kumar K V, Zhou C H, Cai G X. Novelbenzmidazole derived naphthalimide triazoles: synthesis, antimicrobial activity and interactions with calf thymus DNA. Sci. China Chem., 2015,58,483-494" 4-Bromo-1,8-naphthalene diic anhydride was prepared by reacting with ammonia water to obtain 5.08 g of intermediate III with a yield of 92.0%; brown solid.
Embodiment 2
[0038] Embodiment 2, the preparation of intermediate IV
[0039]
[0040]In a 100mL round bottom flask, add III (2.76g, 10mmol), chloroacetone (1.39g, 15mmol), potassium carbonate (1.38g, 10mmol), use DMF (dimethylformamide, 10mL) as solvent, 100°C The reaction was stirred, followed by TLC until the end of the reaction, cooled to room temperature (18-25° C.), concentrated, extracted, separated by column chromatography, and dried to obtain 2.07 g of compound IV with a yield of 62.3%.
[0041] Compound IV: white powder; melting point 220–221°C; 1 H NMR (600MHz, CDCl 3 ): δ8.64(d, J=7.1Hz, NAPH-H,1H), 8.59(d, J=8.4Hz, NAPH-H,1H), 8.40(d, J=7.8Hz, NAPH-H,1H ), 8.04(d, J=7.8Hz, NAPH-H, 1H), 7.85(t, J=7.9Hz, NAPH-H, 1H), 5.01(s, NAPH-CH 2 ,2H),2.34(s,-CH 3 ,3H)ppm.
Embodiment 3
[0042] Embodiment 3, the preparation of intermediate V
[0043]
[0044] In a 100mL round bottom flask, the intermediate IV (3.32g, 10mmol) was dissolved in (10mL) glacial acetic acid, and bromine (1.59g, 10mmol) was slowly added dropwise, and the reaction was stirred at 40°C for 30min under temperature control, Afterwards, the temperature was raised to 60°C and the reaction was stirred. TLC was followed until the end of the reaction. An aqueous solution of sodium bisulfite was added to precipitate out. After cooling, it was filtered with suction, dried, separated by column chromatography, and dried to obtain 2.16 g of compound V with a yield of 52.6%.
[0045] Compound V: white solid; melting point 223–225°C; 1 H NMR (600MHz, DMSO-d 6 ): δ8.60(dd, J=6.5, 5.6Hz, NAPH-H, 2H), 8.36(d, J=7.6Hz, NAPH-H, 1H), 8.26(d, J=7.8Hz, NAPH-H ,1H),8.04(t,J=7.9Hz,NAPH-H,1H),5.15(s,NAPH-CH 2 ,2H),4.64(s,-CH 2 Br, 2H) ppm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inhibitory activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com