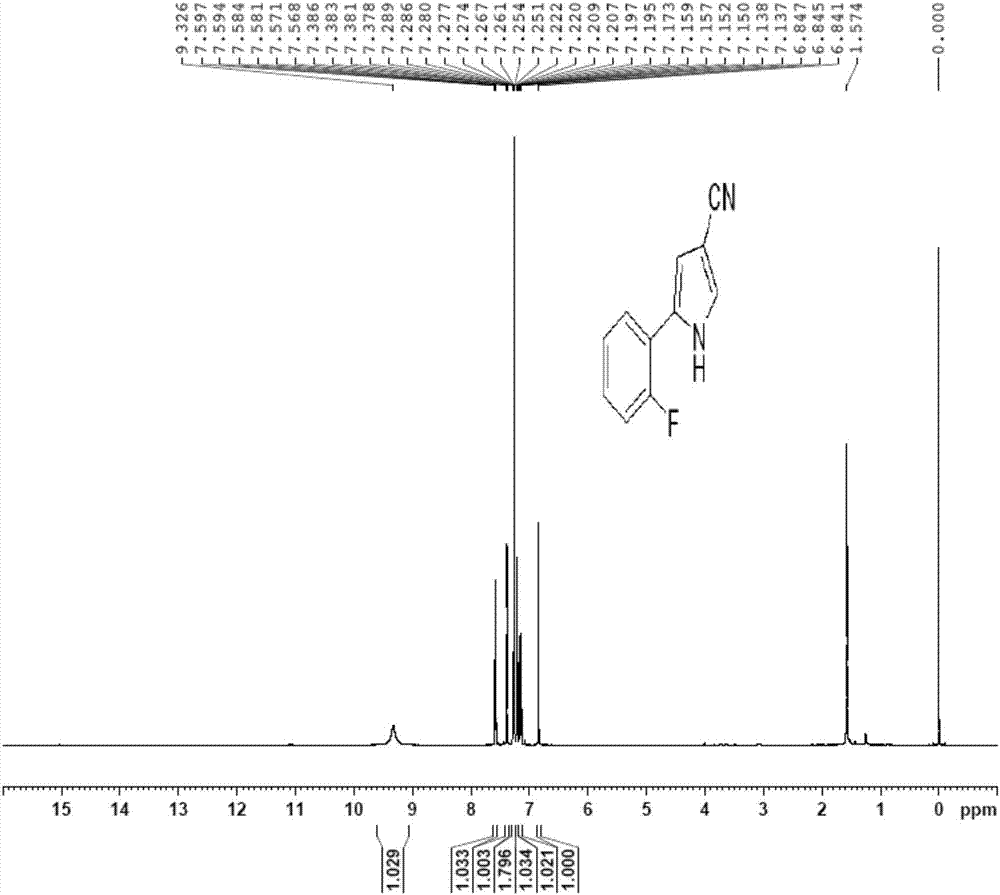
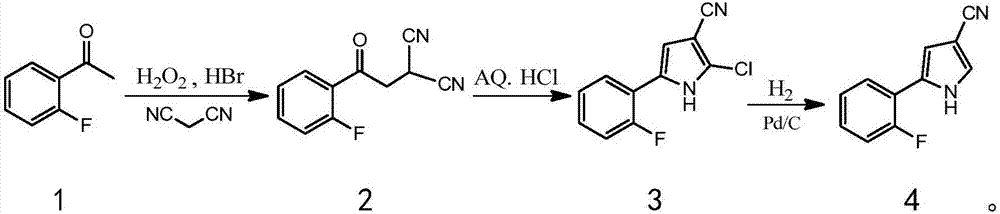
Vonoprazan fumarate key intermediate and preparation method thereof
A technology of vonorazan fumarate and its intermediates, which is applied in the field of organic synthesis, can solve the problems of complex preparation reaction routes, many post-processing procedures, and increased product costs, and achieve simple processes, reduced difficulty, risk, and cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] The preparation method of the key intermediate of vonoprazan fumarate of the present invention mainly includes 3-step procedures, the process is simple, the post-treatment is simple, no toxic reactants and unstable reactants are used, and the difficulty and danger of industrial operation are reduced. The use of catalyst is small, the cost is low, the yield is high, and it is suitable for large-scale production.
[0080] Optionally, the preparation method of the compound 2 comprises:
[0081] Part of the solvent, HBr and hydrogen peroxide are prepared into solution A for subsequent use;
[0082] Add the solution A to the mixed solution of o-fluoroacetophenone and the rest of the solvents to carry out the reaction. After the reaction, add water, then add the reducing agent, stir and separate the layers, and the obtained organic phase is washed and set aside;
[0083] Add malononitrile to the organic phase, stir to dissolve completely, then add alkali to react;
[0084] ...
Embodiment 1
[0167] A preparation method for the key intermediate of vornorazan fumarate, comprising the steps of:
[0168] 1. Preparation of malononitrile condensate:
[0169] First, 3.5 kg of ethyl acetate was added to the reaction bottle; at room temperature, under magnetic stirring, 1.8 kg of HBr aqueous solution with a mass fraction of 47% and 750 g of hydrogen peroxide were added to form solution A, which was sealed for later use;
[0170]Add 1.4 kg of o-fluoroacetophenone and 5.5 kg of ethyl acetate into the reaction flask, seal and stir at room temperature until clear; add the above solution A dropwise at 20°C, and react within this temperature range for 4 hours after the dropwise addition; determine the reaction After completion, lower the temperature to 15°C, control the temperature below 25°C, add 7 kg of water dropwise, then add 250 grams of sodium sulfite, stir for 20 minutes, and separate layers; the organic phase is washed with saturated sodium bicarbonate and saturated sodi...
Embodiment 2
[0178] A preparation method for the key intermediate of vornorazan fumarate, comprising the steps of:
[0179] 1. Preparation of malononitrile condensate:
[0180] First, add 4 kg of ethyl acetate to the reaction bottle; at room temperature, under magnetic stirring, add 2 kg of HBr aqueous solution with a mass fraction of 47% and 900 g of hydrogen peroxide to configure solution A, and seal it for later use;
[0181] Add 1.4 kg of o-fluoroacetophenone and 6 kg of ethyl acetate into the reaction bottle, seal and stir at room temperature until clear; add the above solution A dropwise at 30°C, and react within this temperature range for 3 hours after the dropwise addition; determine the reaction After completion, lower the temperature to 15°C, control the temperature below 35°C, add 8 kg of water dropwise, then add 300 grams of sodium sulfite, stir for 30 minutes, and separate layers; the organic phase is washed with saturated sodium bicarbonate and saturated sodium chloride solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com