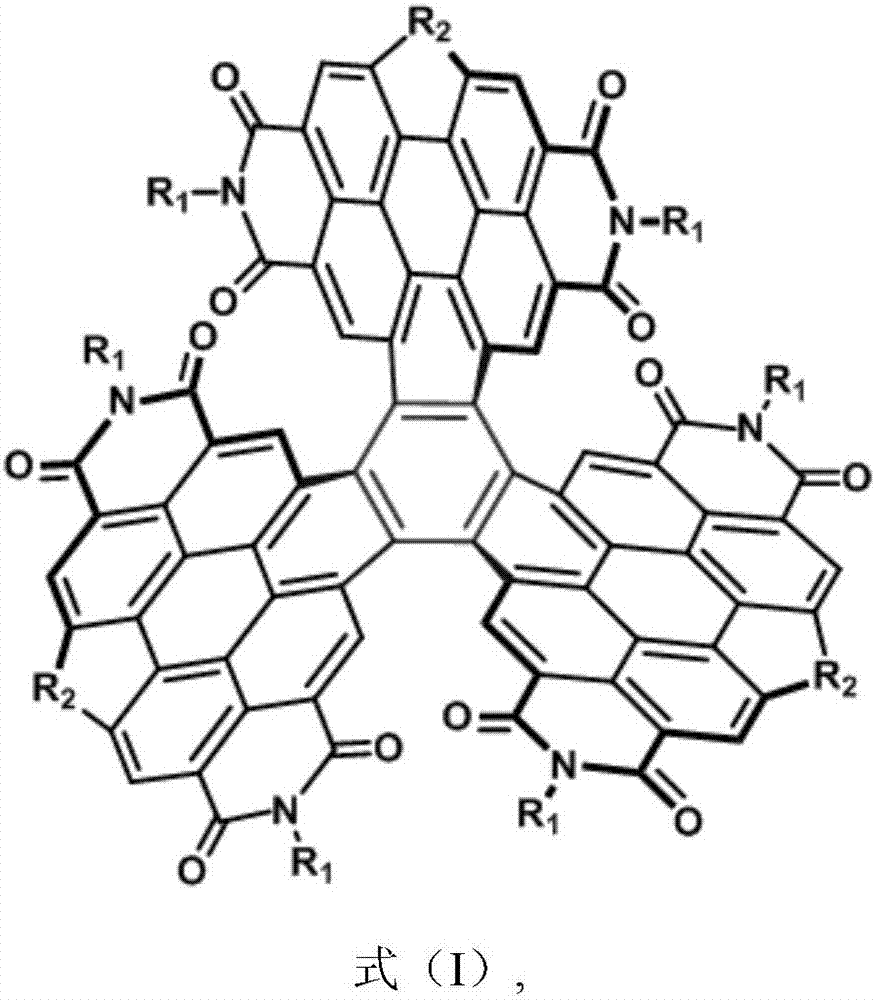
Gear type heterocyclic tripolyperylene tetracarboxylic acid diimide compound and preparation method and application thereof
A polyperylene diimide and gear type technology is applied in the field of gear type heterocyclic trimer perylene diimide compounds and their preparation, and achieves the effects of inhibiting force, shortening synthesis steps and improving photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation of embodiment 1 target product 1
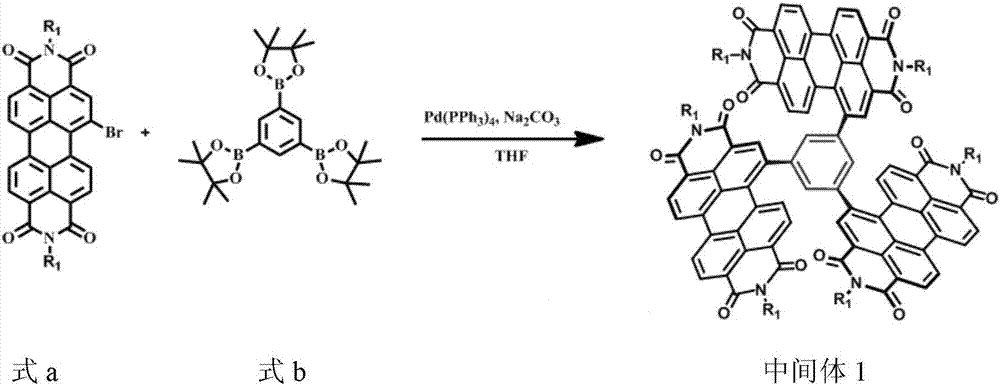
[0047] (1) Preparation of Intermediate 1
[0048] In a glass tube, add monobromoperyleneimide (compound of formula a) (546g, 0.702mmol), 1.3.5-benzenetriborate (compound of formula b) (100mg, 0.219mmol), tetrahydrofuran 8ml and 2M carbonic acid Sodium aqueous solution 4ml, mixed with nitrogen blowing for 30min, then under argon, tetrakis(triphenylphosphine) palladium (35mg, 0.026mmol) was added, the mixture was heated to reflux for 48h, then cooled to room temperature, 25ml of water was added, and dichloro Methane (2 × 25ml) was extracted, dried over anhydrous magnesium sulfate, suction filtered, and the solvent was removed. The crude product was purified through a chromatographic column to obtain an orange solid that was Intermediate 1 (367mg, yield 80%). The reaction equation was as follows:
[0049]
[0050] in,
[0051] The structural confirmation data of intermediate 1 are as follows: 1 H NMR (400MHz, CDCl ...
Embodiment 2
[0071] The preparation of embodiment 2 target product 2
[0072] The chemical structural formula of the target product 2 that the present embodiment obtains is as follows:
[0073]
[0074] in,
[0075] The preparation method of target product 2 is the same as that of Example 1, only the S powder in step (4) is replaced by Se powder, and other conditions remain unchanged.
[0076] The structure confirmation data of the target product 2 are as follows: 1 H NMR (400MHz, CDCl 3 )δ=9.94(s,6H),9.83(s,6H),5.16(m,6H),2.15-2.09(m,12H),1.95(m,12H),1.30-1.24(m,72H),0.79 (s,36H); 13 C NMR (100MHz, CDCl 3 ): δ=164.77, 164.21, 142.05, 134.67, 130.99, 128.94, 128.40, 127.42, 124.18, 123.82, 122.38, 122.16, 55.03, 32.24, 31.53, 31.38, 26AL.53, 22.7783, 13% (MS ):C 144 h 150 N 6 o 12 Theoretical value of Se3: 2392.88791; measured value: 2392.88783.
[0077] The photoelectric conversion performance test of target product 2 is identical with embodiment 1, and the photovoltaic pe...
Embodiment 3
[0079] (1) A mixture of monobromoperyleneimide and 1,3,5-benzenetriborate with a molar ratio of 3:1 is mixed and dissolved in tetrahydrofuran and aqueous sodium carbonate solution by argon blowing, wherein the concentration of sodium carbonate 2mol / L, the volume ratio of tetrahydrofuran and sodium carbonate aqueous solution is 1.8:1, then under argon gas, the catalyst is tetrakis (triphenylphosphine) palladium, mixed and heated to reflux to obtain orange solid intermediate 1;
[0080]
[0081] where R 1 for
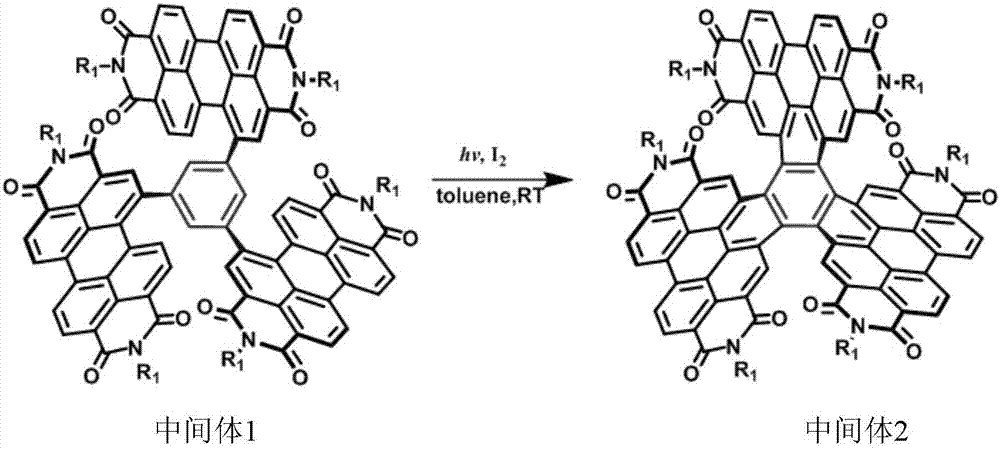
[0082] (2) Intermediate 1, I 2 The mixture with toluene was irradiated under a mercury lamp, then extracted with saturated aqueous sodium sulfite, washed, dried, filtered, and chromatographically purified to obtain dark red solid intermediate 2;
[0083]
[0084] where R 1 for
[0085] (3) Add the dichloromethane solution of fuming nitric acid to intermediate 2 and dichloromethane to react for 11h, wherein the volume ratio of fuming nitric acid to dichlorome...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Short circuit current | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com