Method of establishing liquid chromatography-mass spectrometry (LC-MS) analysis of royal jelly sensitized protein
A royal jelly protein, liquid chromatography-mass spectrometry technology, applied in the field of liquid mass spectrometry analysis of royal jelly allergenic proteins, can solve the problems of poor linear range, difficulty in large-scale promotion, high detection limit, etc., and achieve high accuracy and repeatability Good, highly specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
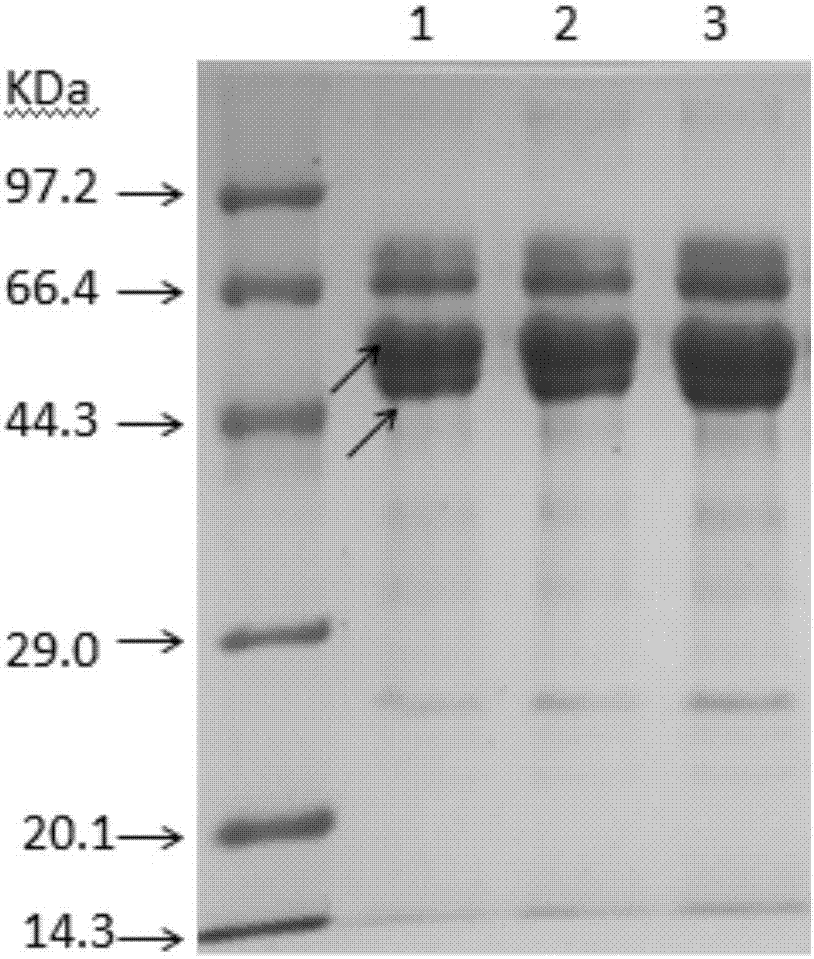
[0053] Extraction of protein in RJ
[0054] Take an appropriate amount of pure RJ sample and dilute it with ultrapure water at a mass ratio of 4:5 (RJ / Water), extract overnight at 4°C, then centrifuge at 12000g for 30min; after centrifugation, take the supernatant and continue centrifuging for 20min, then take the upper The supernatant was frozen at -18°C for later use. The protein content in the supernatant was determined by the Coomassie brilliant blue G-250 method.
[0055] Pretreatment of MRJP1 quantitative samples
[0056] Take 50 μL of the above supernatant and wash with 40 mM NH 4 HCO 3 Dilute the solution to 1 mL, take 900 μL of the diluted solution (at this time, the protein content is about 2.5 mg), first add a certain amount of internal standard peptide standard solution, then add 10 μL of 500 mM dithiothreitol (DTT), and heat in a water bath at 56 °C 30min; after cooling, add 30μL of 500mM iodoacetamide (IAA), and react at room temperature in the dark for 30min...
Embodiment 2
[0108] The LC-MS / MS method is as follows:
[0109] Chromatographic conditions:
[0110] Chromatographic column: Acquity BEH C18 column (2.1mm x 100mm, 1.7μm); column temperature: 40°C; injection volume: 10μL; mobile phase A: 0.1% formic acid / water solution; mobile phase B: 0.1% formic acid / acetonitrile solution; flow rate: 0.3mL / min; gradient elution, elution program: within 10min, 97%A to 60%A; within 0.1min, 60%A to 100%A, and maintain 100%A for 1min, then within 1min To 97% A. Finally, the column was equilibrated under the condition of 97% A for 3 minutes.
[0111] Mass Spectrometry Conditions:
[0112] Electrospray ion source ionization mode: ESI+; capillary voltage: 4.0kV; cone voltage: 25V; extractor voltage: 2.5V; entrance lens voltage: 0.2V; ion source temperature: 110°C; desolvation temperature: 360°C; desolvation Air flow: 550L / h; Cone blowback airflow: 45L / h; Scanning mode: Multi-ion reaction monitoring mode (MRM).
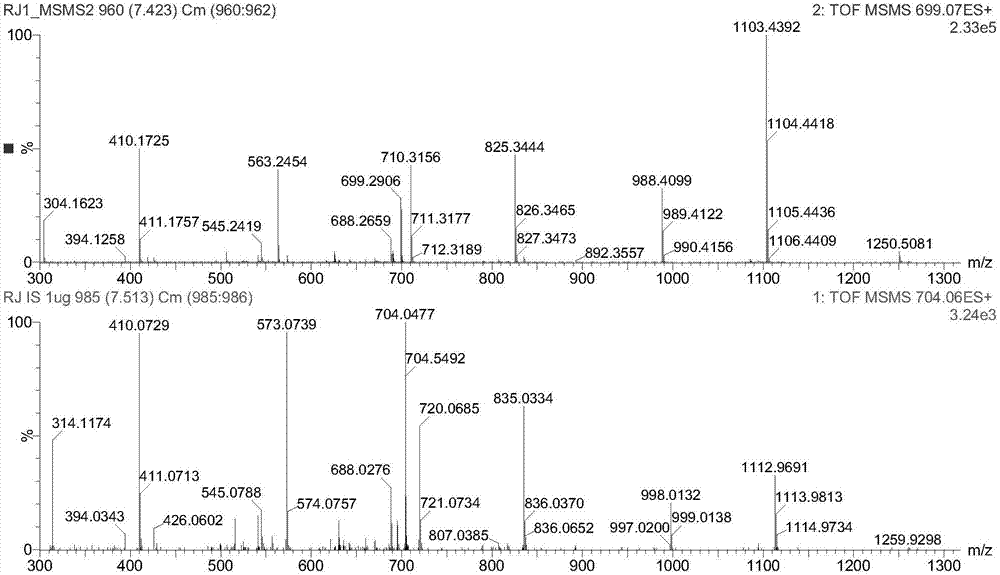
[0113] According to the experimental results,...
Embodiment 3
[0115] 1 mg peptide standard, first dissolved in 125 μL of acetonitrile into a suspension, and then added 500 μL of water.
[0116] At this time, the dissolution of the standard is not complete, and the quantitative result is inaccurate, the detection limit rises, and the sensitivity drops.
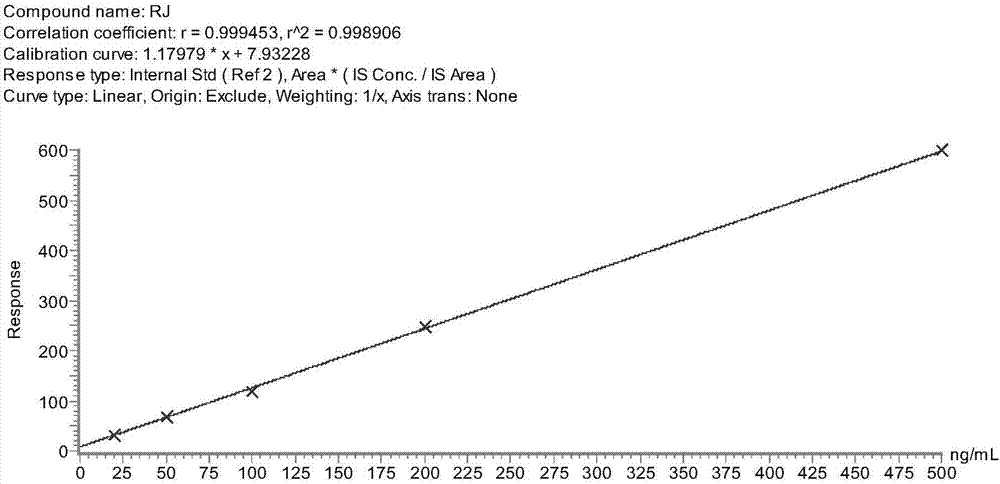
[0117] It can be seen that the quantitative method for allergenic proteins invented by us has strong specificity, high sensitivity (the detection limit LOD of MRJP1 in the method is 7.04 μg / g, the limit of quantification LOQ is 23.07 μg / g), good repeatability (relative standard deviation RSD range 4.6~6.3%), high accuracy (recoveries of three spiked concentrations are 88.65%, 90.11% and 87.26%), etc., can effectively carry out accurate quantitative analysis of certain allergenic proteins in actual samples .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com