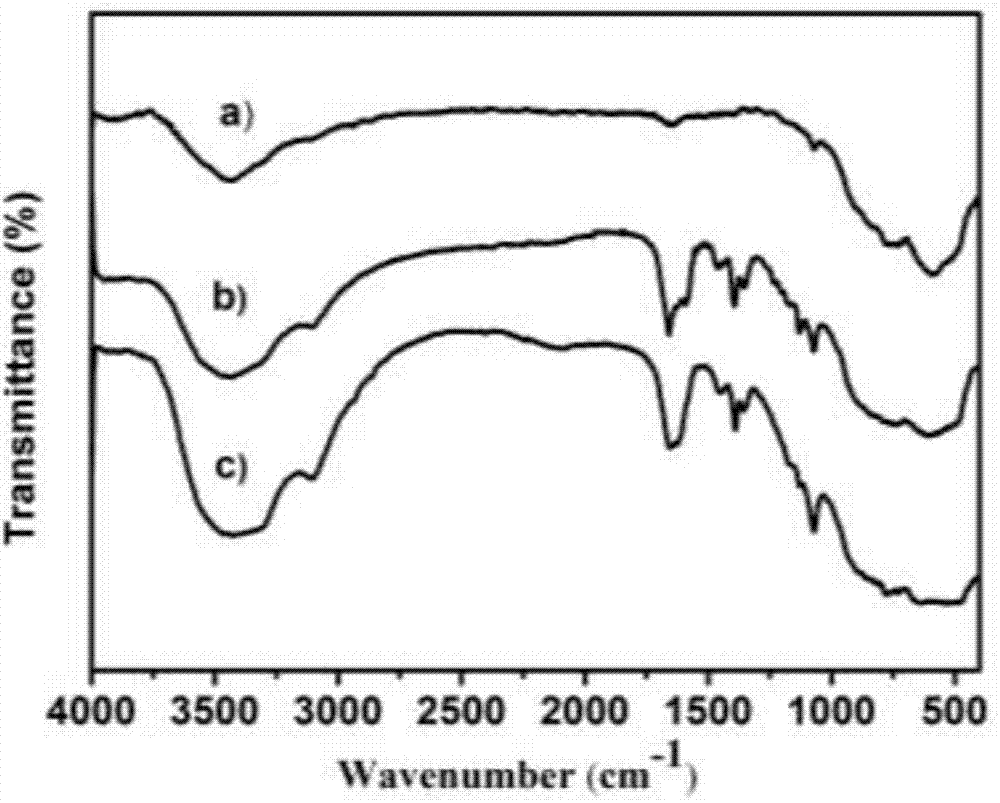
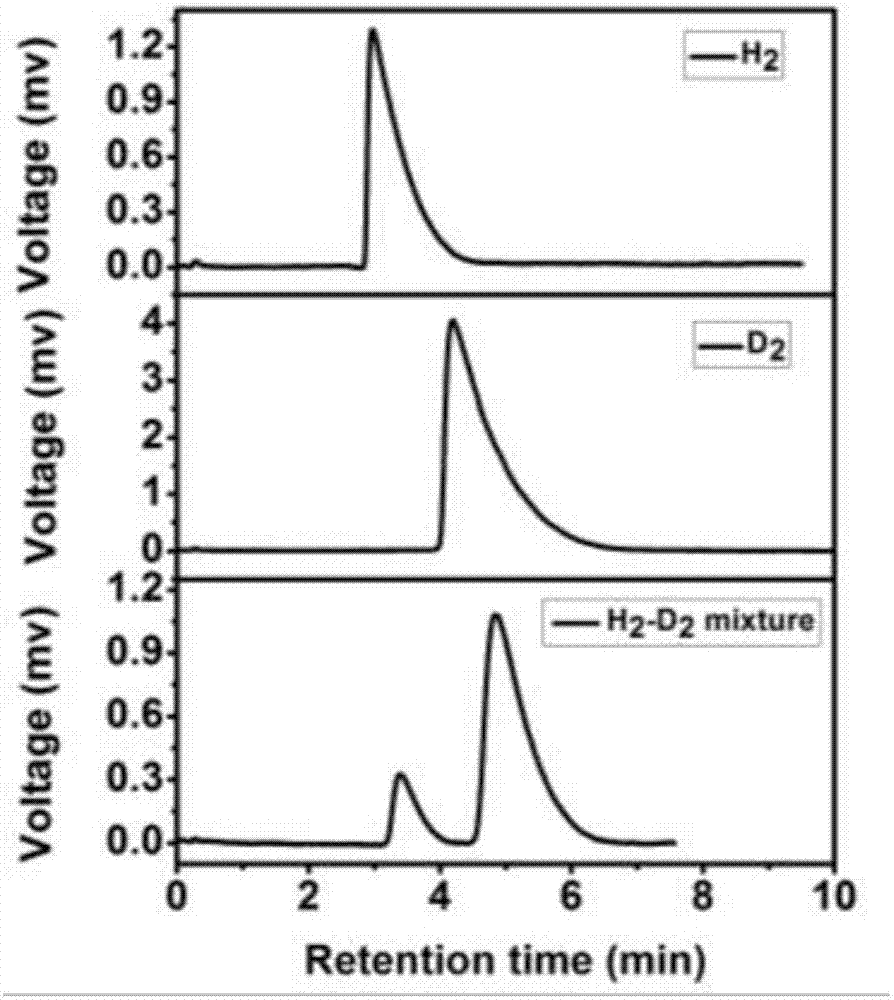
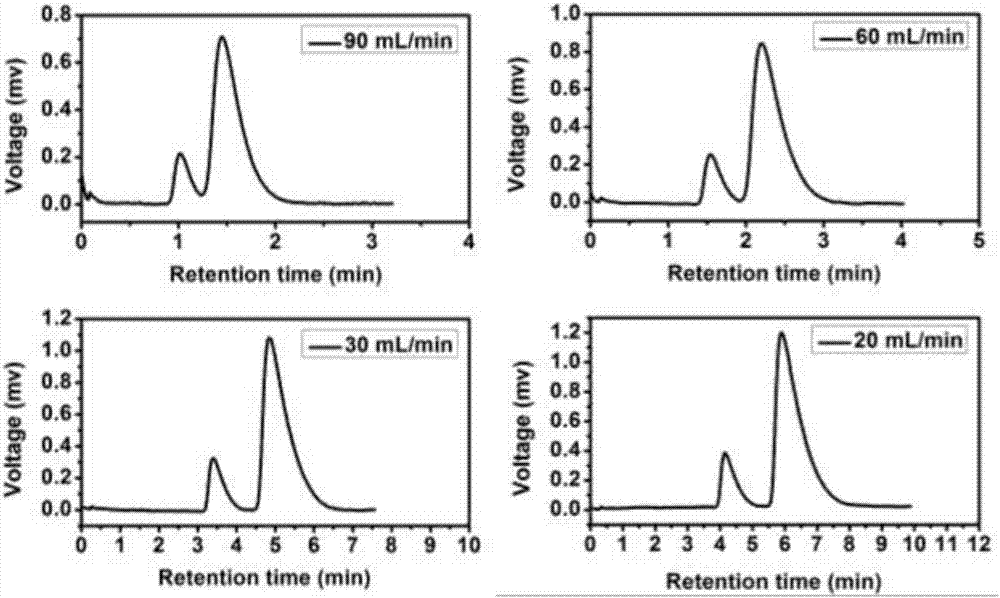
Gas-chromatography filler capable of separating and analyzing hydrogen isotopes and preparation method of gas-chromatography filler
A hydrogen isotope and gas chromatography technology, which is applied in the field of loaded chromatographic packing and its preparation, can solve the problems of high energy consumption, clogging of chromatographic columns, and affecting the separation effect, and achieve accurate quantitative analysis, short separation time and good separation effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) 3.0g of γ-Al with a particle size of 80-100 mesh 2 o 3 Add to a solution consisting of 0.5mmol copper perchlorate hexahydrate, 0.5mmol sodium pyrazine-2,3-dioate, 0.625mmol pyrazine and 20mL deionized water, and immerse for 2h;
[0037] (2) Take the product in step (1) out of the soaking solution, rinse it with deionized water, and then dry it in an oven at 110°C for 30 minutes;
[0038] (3) Steps (1)-(2) are used as a cyclic process, and the product obtained in step (2) is substituted for γ-Al in step (1) 2 o 3 Repeat this cycle for 7 times.
[0039] (4) Add 3.00 g of the product obtained in step (3) to MnCl composed of 3.58 mmol of manganese chloride tetrahydrate and 20 mL of deionized water 2 The solution was immersed for 2 hours; transferred together to a 100mL round bottom flask, the solvent was evaporated to dryness with a rotary evaporator, and the product was dried at 100°C for 10 hours to obtain a supported composite material.
Embodiment 2
[0041] (1) 3.00g pre-activated γ-Al 2 o 3 Dipping in the following order: a) 100mL 10mM trimesic acid ethanol solution, 40min, b) 20mL ethanol, 5min, c) 100mL 10mM copper acetate trihydrate ethanol solution, 20min, d) 20mL ethanol, 5min
[0042] (2) In the step (1), a) to d) constitute one cycle, and thus cycle impregnation 4 times to obtain a supported composite material, which is then dried at 100° C. for 30 min.
[0043] (3) Add 3.00 g of the sample in step (2) to MnCl composed of 3.58 mmol of manganese chloride tetrahydrate and 20 mL of deionized water 2 The solution was immersed for 2 hours; transferred together to a 100mL round bottom flask, the solvent was evaporated to dryness with a rotary evaporator, and the product was dried at 100°C for 10 hours to obtain a supported composite material.
Embodiment 3
[0045] (1) Add 0.182mmol 2,5-dihydroxyterephthalic acid and 0.613mmol nickel nitrate hexahydrate to 15mL (volume ratio 1:1:1) of DMF-ethanol-water mixed solvent, sonicate until completely dissolved ;
[0046] (2) Transfer the solution in step (1) to a 23mL hydrothermal reaction kettle with PTFE lining, and add 0.60g of 80-100 mesh γ-Al at the same time 2 o 3 , at 1.5°C·min -1 After rising to 100°C, keep it for 24 hours, then cool down to room temperature naturally;
[0047] (3) The product obtained in step (2) is separated from the mother liquor by decantation, and the sample is soaked in 15mL of methanol solvent for solvent exchange, and solvent exchange is carried out 4 times within 48h to exchange the DMF adsorbed in the composite material, and finally in 100 Dry in a drying oven at ℃ for 5 hours to remove methanol to obtain the product.
[0048] (4) Add 3.00 g of the product in step (3) to MnCl composed of 3.58 mmol of manganese chloride tetrahydrate and 20 mL of deion...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com