Preparation method of positive material sodium hexafluoroferrate applicable for sodium or lithium ion batteries and clad material thereof
A technology of sodium hexafluoroferrate and lithium ion battery, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problem of low electrical conductivity of sodium hexafluoroferrate material, impurities in sodium hexafluoroferrate material, and cycle performance. To achieve the effect of uniform and controllable particle size, good fluidity and good crystallization performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
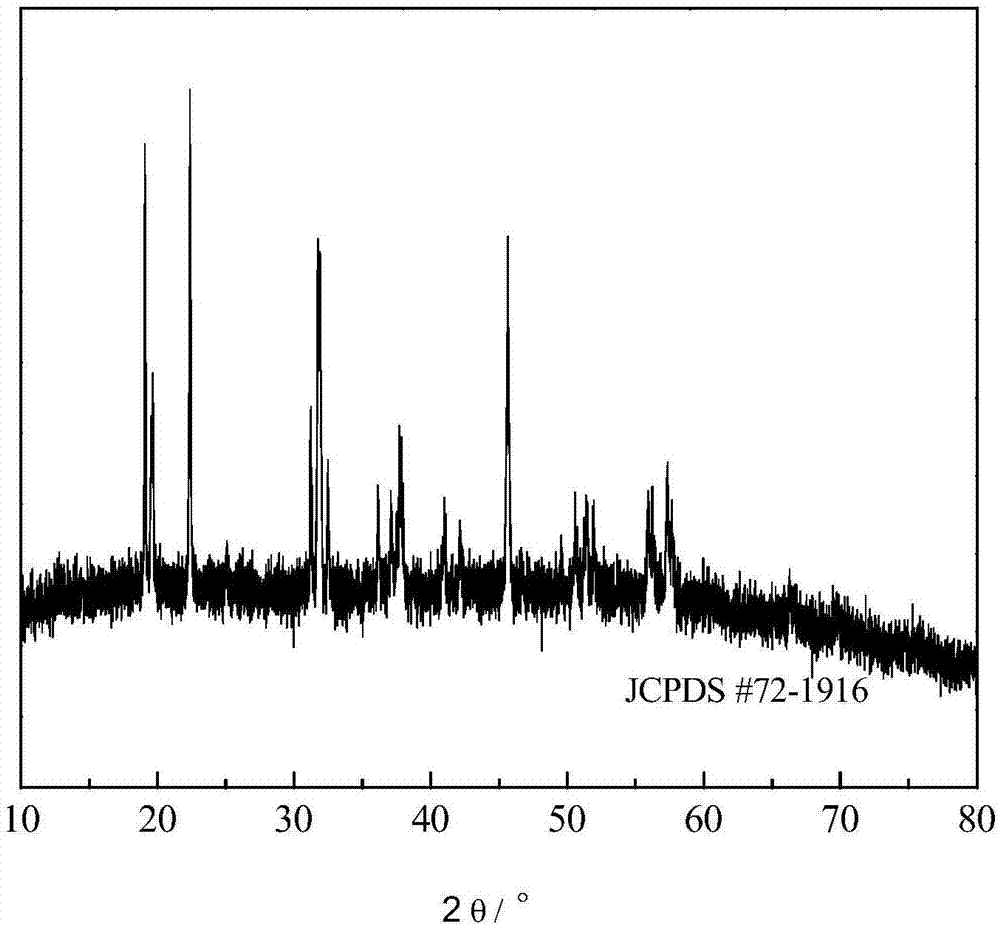
[0035] Example 1: Weigh about 100 g of sodium sulfate, ferric sulfate and ammonium fluoride at a molar ratio of Na:Fe:F=3:1:6, dissolve them in deionized water at 10°C, and prepare sodium sulfate solution, sulfuric acid Iron solution and ammonium fluoride solution, the first two solutions are mixed to obtain a mixed solution of sodium sulfate and ferric sulfate. Under the action of mechanical stirring at a speed of 200rpm, ammonium fluoride solution was added to the mixed solution. After reacting for 3 hours, stop stirring, let stand for 5 hours, filter, wash with deionized water for 3 times, and vacuum dry at 120°C for 4 hours to obtain Sodium hexafluoroferrate material.
[0036] Put carbon black and sodium hexafluoroferrate into the ball milling tank at a mass ratio of 10:90, and add absolute ethanol. After ball milling at a speed of 400rpm for 5h, dry the product at 60°C in an air atmosphere for 10h, and crush Grading to obtain the carbon black-coated sodium hexafluoroferr...
Embodiment 2
[0037] Example 2: Weigh about 100 g of sodium nitrate, ferric nitrate and hydrofluoric acid at a molar ratio of Na:Fe:F=3:1:6, dissolve them in deionized water at 25° C., and prepare sodium nitrate solution, nitric acid Iron solution and ammonium fluoride solution, the first two solutions are mixed to obtain a mixed solution of sodium nitrate and ferric nitrate. Under the action of mechanical stirring at a speed of 300rpm, add the mixed solution and the ammonium fluoride solution into the reactor at the same time, after reacting for 4 hours, stop stirring, let stand for 8 hours, filter, wash with deionized water 4 times, and dry in vacuum at 100°C 6h, namely sodium hexafluoroferrate material.
[0038]Put carbon nanotubes and sodium hexafluoroferrate into a ball milling tank at a mass ratio of 5:95, and add absolute ethanol. After ball milling at a speed of 500 rpm for 4 hours, dry the product at 60°C in an air atmosphere for 12 hours. After crushing and classifying, the carbo...
Embodiment 3
[0039] Example 3: Weigh about 100 g of sodium fluoride, ferric chloride and ammonium fluoride at a molar ratio of Na:Fe:F=3:1:6, dissolve them in deionized water at 40°C, and prepare sodium fluoride solution, ferric chloride solution and ammonium fluoride solution, the latter two solutions are mixed to obtain a mixed solution of ferric chloride and ammonium fluoride. Under the action of mechanical stirring at a speed of 400rpm, add the mixed solution and sodium fluoride solution into the reactor at the same time. After reacting for 5 hours, stop stirring, let it stand for 10 hours, filter, wash with deionized water for 5 times, and vacuum dry at 90°C. After 10 hours, the sodium hexafluoroferrate material was obtained.
[0040] Add graphene and sodium hexafluoroferrate materials into the ball mill tank at a mass ratio of 2:98, and add acetone. After ball milling at a speed of 600rpm for 3 hours, the product is dried at 80°C for 8 hours in a nitrogen atmosphere, broken and class...
PUM
| Property | Measurement | Unit |
|---|---|---|
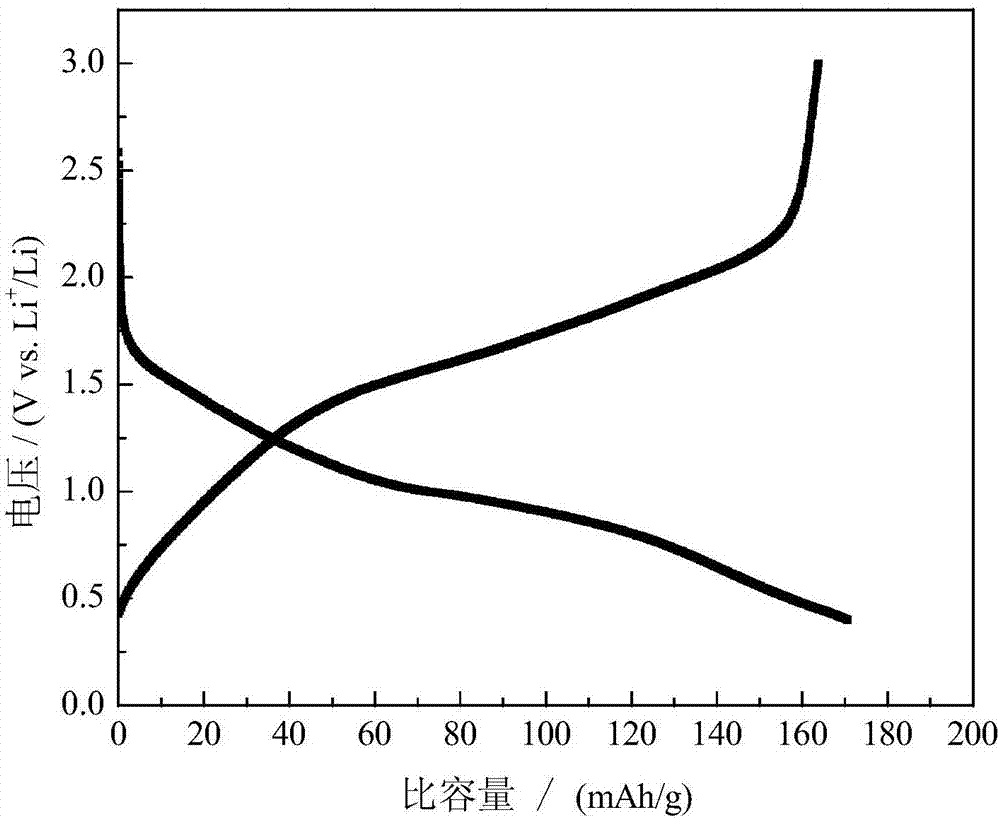
| Charge specific capacity | aaaaa | aaaaa |
| Discharge specific capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com