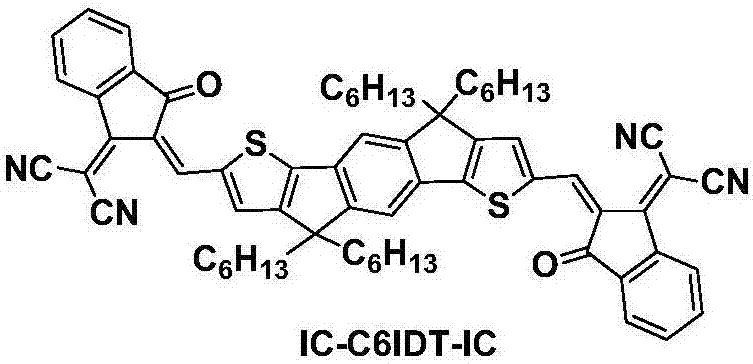
Organic small-molecule donor photovoltaic material containing fluorinated benzotriazole as well as preparation method and application of organic small-molecule donor photovoltaic material
An organic and photoelectric technology, applied in photovoltaic power generation, organic chemistry, silicon organic compounds, etc., can solve the problem of low photoelectric conversion efficiency, and achieve the effect of superior photovoltaic performance, easy purification, and structure determination.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The organic photoelectric compound of this embodiment is composed of a large organic conjugated unit system and an alkane or alkane-like structure, and is a black solid powder with metallic luster. The general chemical structure of the compound is shown in Formula II:
[0064]
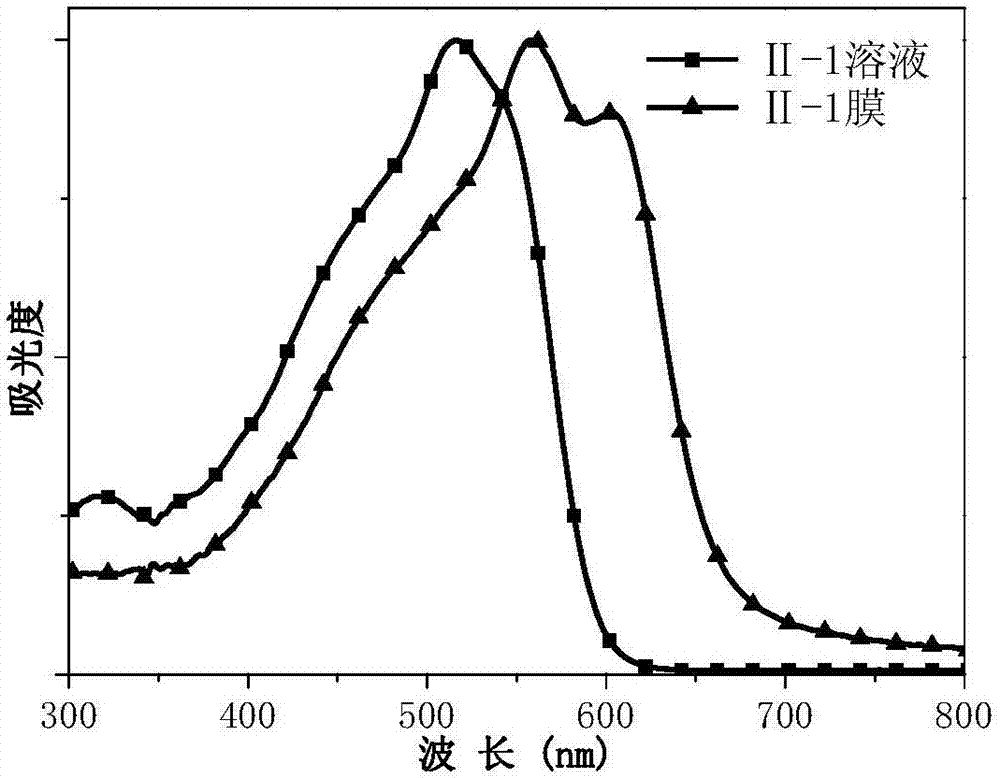
[0065] where: R 6 is 2-ethylhexyl, R 7 It is 2-hexyloctyl, that is, the chemical structural formula of the compound is as shown in formula II-1:
[0066]
[0067] The preparation method of the organic photoelectric compound used in solar cells is prepared by carrying out Knoevenagel condensation reaction of a dialdehyde terminal compound and a terminal precursor compound, comprising the following steps:
[0068] 1) Under argon protection, 5-(7-(5-bromothiophen-2-yl)-5,6-difluoro-2-(2-hexyldecyl)-2H-benzo[d][1 ,2,3] Triazol-4-yl)thiophene-2-carbaldehyde 3.25g (5.0mmol) and 2-ethylhexylthienyl substituted benzodithiophene bistin monomer 2.26g (2.5mmol), 30mL Dried toluene and triphenylpho...
Embodiment 2
[0076] The organic photoelectric compound of this embodiment is composed of a large organic conjugated unit system and an alkane or alkane-like structure, and is a black solid powder with a metallic luster. The general chemical structure of the compound is shown in Formula V below:
[0077]
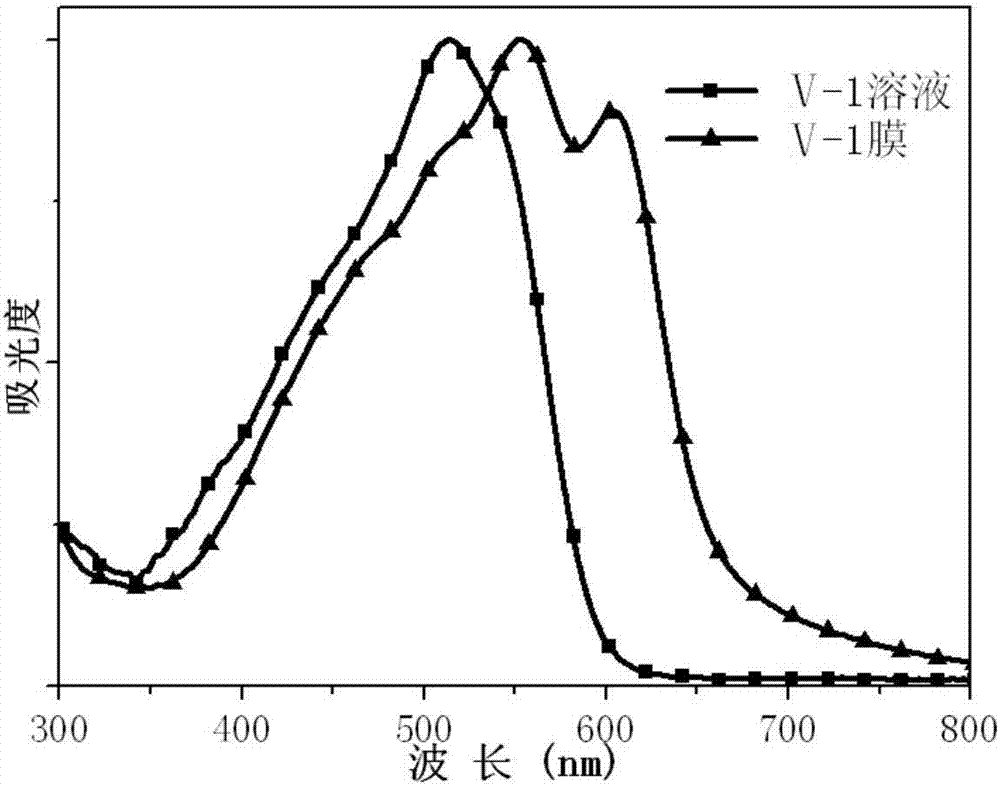
[0078] Among them, R 7 is 2-hexyloctyl, R 8 Be 2-ethylhexyl, that is, the chemical structural formula of the compound is formula V-1:
[0079]
[0080] The preparation method of the organic photoelectric compound for solar cells is basically the same as that of Example 1, except that the benzodithiophene ditin monomer replaced by thiophene ditin monomer is replaced by 2-ethylhexylthiophene ditin monomer, and also Using chloroform as a solvent and piperidine as a catalyst, an organic photoelectric compound is prepared with a yield of 85%.
[0081] 1 H NMR (400MHz, CDCl3): δ (ppm) 8.21-8.20 (m, 4H), 8.18 (s, 2H), 7.77-7.76 (d, 2H), 7.11-7.10 (m, 4H), 4.75-4.74 ( d,4H),4.17-4.09(m,...
Embodiment 3
[0085] Example 3. Film-forming and solubility tests of small molecules of the present invention.
[0086] Place the compounds shown in formula II-1 and formula V-1 prepared in Example 1 and Example 2 in several common organic solvents, such as chlorobenzene, dichlorobenzene, chloroform, toluene, trichlorobenzene, methanol Wait. The compound was found to have good solubility in chlorinated solvents, but was insoluble in methanol. High-quality films can be prepared by spin-coating the trichloride solution of the compound on a glass slide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com