Quaternary ammonium salt with small molecular weight and preparation method and application thereof
A small molecular weight, quaternary ammonium salt technology, applied in the field of compounds carrying nucleic acid drugs, to achieve the effect of low toxicity and improved release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
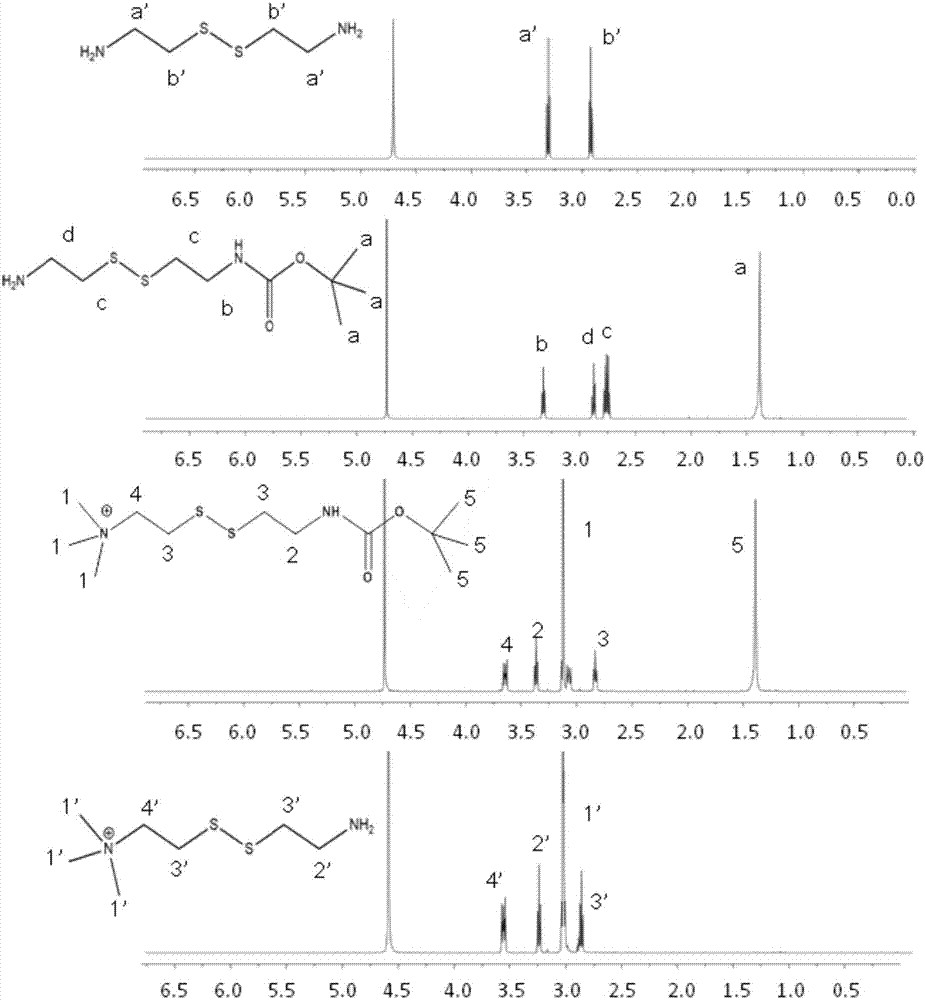
[0046] Embodiment 1: synthetic N, N, N-trimethylcystamine trifluoroacetate
[0047]1) Weigh 200 mg of cystamine dihydrochloride (referred to as cystamine) and dissolve in 10 ml of methanol, and add 386 μl of triethylamine; dissolve 192.5 mg of di-tert-butyl dicarbonate (BOC anhydride) in 2 ml of methanol, gradually Add it dropwise to methanol solution of cystamine dihydrochloride, stir at room temperature for 30 minutes, and evaporate methanol to dryness by rotary evaporation. Continue to add 50ml of sodium dihydrogen phosphate solution (NaH 2 PO 4 , 1M), washed twice with ether to remove the product that both ends are connected to BOC anhydride, using NaOH (1M) to adjust the pH of the solution to 9, extracting with ethyl acetate to obtain the cystamine protected by BOC at one end, rotary steaming The ethyl acetate layer was dried to give a white product (designated cystamine-BOC).
[0048] 2) Weigh 50 mg of the above product, dissolve it in 1 ml of acetonitrile, add 64.6 m...
Embodiment 2
[0053] Embodiment 2: Synthetic N, N, N-trimethylcystamine iodide salt
[0054] (1) Dissolve 500 mg of cystamine dihydrochloride (referred to as cystamine) in 10 ml of methanol, and add 965 μl of triethylamine; react for 30 minutes, dissolve 223 mg of benzyl chloroformate (CBZ) in 2 ml of methanol, Add it dropwise to methanol solution of cystamine dihydrochloride, stir at room temperature, observe the reaction progress by thin-layer chromatography, and evaporate the methanol to dryness after the raw material point disappears. Continue to add hydrochloric acid solution (HCl, 1M), wash twice with ether to remove the product with CBZ anhydride attached to both ends, use NaOH (1M) to adjust the pH of the solution to 9, and extract with ethyl acetate to obtain CBZ protected at one end. Cystamine, and the ethyl acetate layer was evaporated to dryness by rotary evaporation to obtain a white product (referred to as cystamine-CBZ).
[0055] (2) Weigh cystamine-CBZ 39mg, add 64.6mg K 2...
Embodiment 3
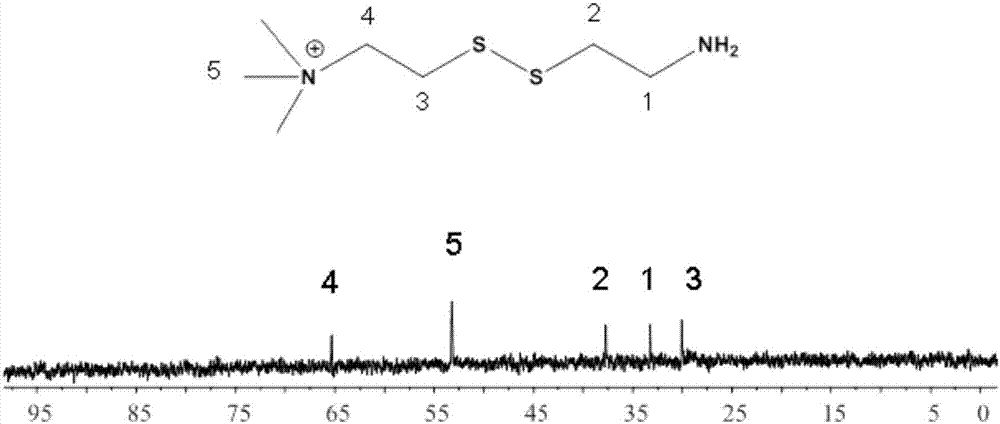
[0057] Embodiment 3: synthetic N, N, N-trimethylcystamine chloride salt
[0058] In this example, 1 ml of saturated ethyl acetate (3M) hydrochloric acid was added to remove BOC, reacted at room temperature for 30 minutes, and the solvent was spin-dried, and the rest of the steps were the same as in Example 1 to prepare solid N,N,N-trimethylcystamine chloride Salt.
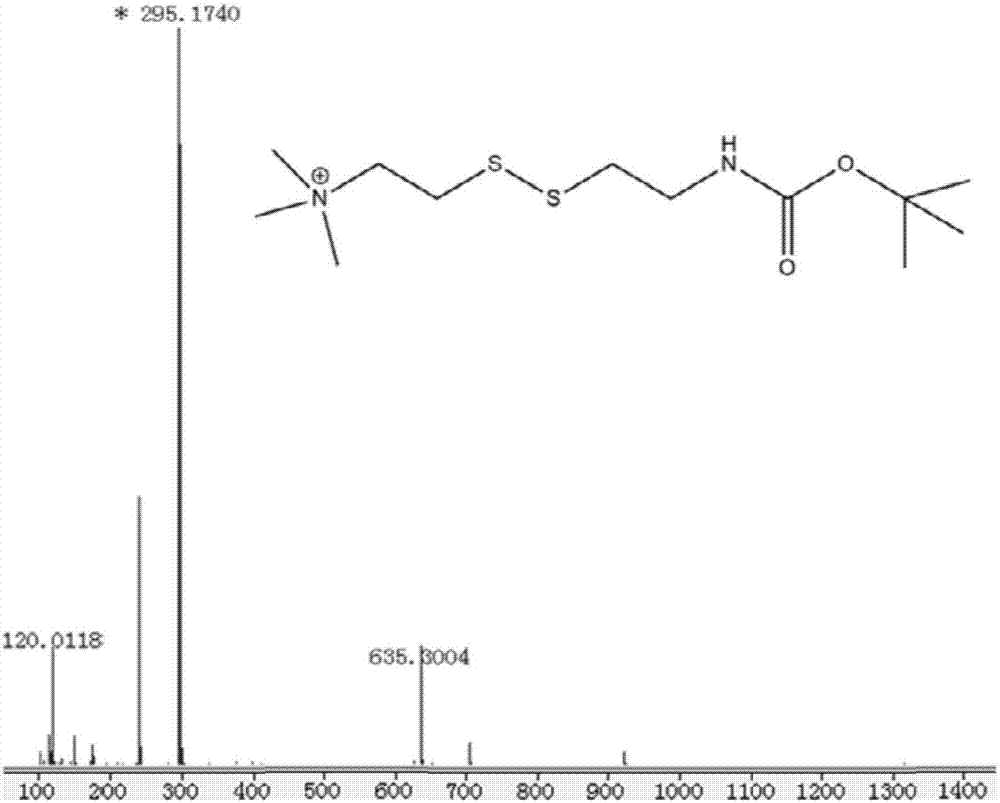
[0059] The result shows: carry out mass spectrometric characterization for N,N,N-trimethylcystamine iodonium salt-BOC and N,N,N-trimethylcystamine chloride salt in embodiment 3, such as Figure 3-4 shown. Molecular ion peak of N,N,N-trimethylcystamine iodide-BOC ([M+H] + ) is 295.17, and the molecular ion peak of N,N,N-trimethylcystamine chloride ([M+H] + ) is 195.10, which proves that N,N,N-trimethylcystamine chloride has been successfully synthesized.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com