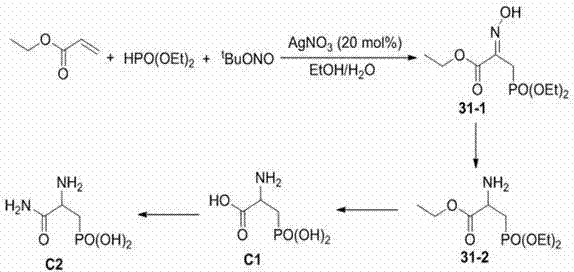
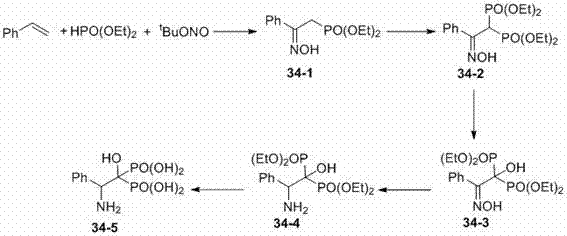
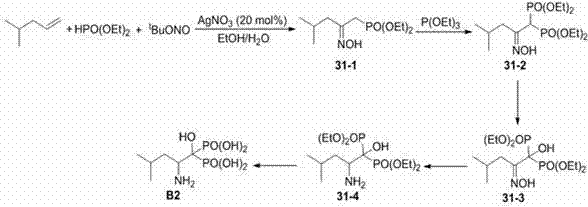
Beta-hydroxyimino phosphono derivatives and preparation methods thereof
A technology of hydroxyliminophosphono and hydroxyliminodiphosphono, which is applied in the field of β-hydroxyiminophosphono derivatives and its preparation, and can solve the problem of cumbersome reaction steps, harsh reaction conditions, and multiple reaction steps. and other problems, to achieve the effect of easy-to-obtain raw materials, short reaction time and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Synthesis of 2-phenyl-2-hydroxyimino ethyl diphenoxyphos
[0047] Using styrene and diphenylphosphine oxide as raw materials, the reaction steps are as follows:
[0048] Add styrene (0.042 g, 0.4 mmol), diphenoxyphos (0.081 g, 0.4 mmol), tert-butyl nitrite (0.041 g, 0.4 mmol), silver nitrate (0.07 g, 0.04 mmol) in the reaction flask, Methanol (2.5 mL), react at 10°C;
[0049] TLC tracking reaction until complete completion;
[0050] The crude product obtained after the reaction was separated by column chromatography (ethyl acetate:petroleum ether=1:1) to obtain the target product (yield 80%). The analytical data of the product are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 11.47 (s, 1H), 7.80 –7.73 (m, 4H), 7.65 – 7.55 (m, 2H), 7.57 – 7.51 (m, 2H), 7.50 –7.45(m, 4H),7.32 – 7.25 (m, 3H ), 4.11 (d, J = 15.4 Hz, 2H).
Embodiment 2
[0051] Example 2: Synthesis of 2-(2-methylphenyl)-2-hydroxyiminoethyl diphenoxyphos
[0052] With 2-methylstyrene and diphenylphosphine oxide as raw materials, the reaction steps are as follows:
[0053] Add 2-methylstyrene (0.047 g, 0.4 mmol), diphenoxyphos (0.081 g, 0.4 mmol), tert-butyl nitrite (0.082 g, 0.8 mmol) and silver nitrate (0.14 g, 0.08 mmol), ethanol (2.5 mL), react at room temperature;
[0054] TLC tracking reaction until complete completion;
[0055] The crude product obtained after the reaction was separated by column chromatography (ethyl acetate:petroleum ether=1:1) to obtain the target product (yield 75%). The analytical data of the product are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 10.85 (s, 1H), 7.80 –7.71 (m, 4H), 7.53 – 7.37 (m, 6H), 7.12 – 6.97 (m, 4H), 3.78 (d, J = 11.0 Hz, 2H), 2.07 (s, 3H).
Embodiment 3
[0056] Example 3: Synthesis of 2-(4-methylphenyl)-2-hydroxyiminoethyl diphenoxyphos
[0057] With 4-methylstyrene, diphenylphosphine oxide as raw material, its reaction steps are as follows:
[0058] Add 4-methylstyrene (0.047 g, 0.4 mmol), diphenoxyphos (0.081 g, 0.4 mmol), tert-butyl nitrite (0.123 g, 1.2 mmol) and silver nitrate (0.21 g, 0.12 mmol), acetonitrile (2.5 mL), react at 30°C;
[0059] TLC tracking reaction until complete completion;
[0060] The crude product obtained after the reaction was separated by column chromatography (ethyl acetate:petroleum ether=1:1) to obtain the target product (yield 82%). The analytical data of the product are as follows: 1 H NMR (400 MHz, CDCl 3 ): δ 11.37 (s, 1H), 7.81 –7.72 (m, 4H), 7.55 – 7.47 (m, 8H), 7.08 (d, J = 7.5 Hz, 2H), 4.08 (d, J =15.4 Hz, 2H), 2.28 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com