Porous spherical carbon-coated sodium vanadium phosphate composite positive electrode material and preparation method thereof
A technology of carbon-coated sodium vanadium phosphate and composite positive electrode materials, which is applied in nanotechnology for materials and surface science, battery electrodes, electrical components, etc., can solve the problems of poor performance of synthetic materials, long production operation time, and preparation process Complicated problems, to achieve excellent electrochemical performance, short cycle, low reaction temperature effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
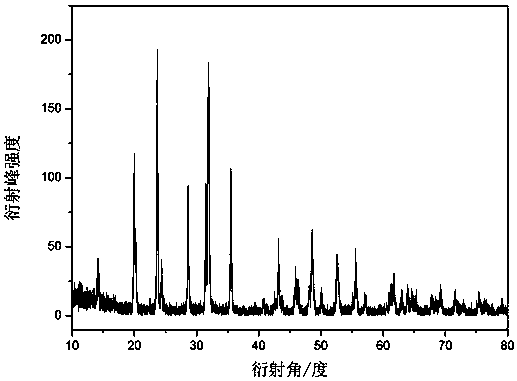
Image
Examples
Embodiment 1
[0046] (1) Dissolve 4mmol (0.4679g) of ammonium metavanadate and 6mmol (0.7564g) of oxalic acid dihydrate in 20mL of deionized water, heat and stir at 80°C until the solution turns blue, then add 6mmol (0.7199g) Sodium dihydrogen phosphate, stirred and dissolved to obtain 20mL blue mixed solution;
[0047] (2) Add 30 mL of N,N-dimethylformamide (the volume after mixing is 50 mL) to 20 mL of the blue mixed solution obtained in step (1), and place it in a 100 mL polytetrafluoroethylene-lined stainless steel sealed reaction kettle , at 180°C, react for 20h, cool, centrifuge, wash the cross-precipitate with ethanol and water for 3 times, and then dry at 80°C for 24h to obtain the precursor powder;
[0048] (3) Mix the precursor powder obtained in step (2) with 0.1982g glucose evenly, then place it in a high-purity argon atmosphere, sinter at 750°C for 10h, then cool to room temperature with the furnace, and coat it with porous spherical carbon Sodium vanadium phosphate composite ...
Embodiment 2
[0057] (1) Dissolve 1mmol (0.1820g) vanadium pentoxide and 1mmol (0.1921g) citric acid in 30mL deionized water, heat and stir at 70°C until the solution turns blue, then add 3mmol (0.395g) phosphoric acid (mass fraction 74.4%) and 4mmol (0.16g) sodium hydroxide, stirred and dissolved to obtain 30mL blue mixed solution;
[0058] (2) Add 30mL of n-propanol (60mL volume after mixing) to the 30mL blue mixed solution obtained in step (1), place it in a 100mL polytetrafluoroethylene-lined stainless steel sealed reaction vessel, and react at 160°C 20h, cooling, centrifuging, washing the cross-precipitation with ethanol and water for 4 times, and then drying at 80°C for 20h to obtain the precursor powder;
[0059] (3) Mix the precursor powder obtained in step (2) with 0.0991g of glucose evenly, then place it in a high-purity nitrogen atmosphere, sinter at 750°C for 10h, and then cool to room temperature with the furnace to form a porous spherical carbon-coated phosphoric acid Vanadiu...
Embodiment 3
[0067] (1) Dissolve 4mmol (0.4679g) of ammonium metavanadate and 3mmol (0.1875g) of hydrazine hydrate (mass fraction 80%) in 10mL of deionized water, heat and stir at 90°C until the solution turns green, then add 6mmol (0.7199g) sodium dihydrogen phosphate, stirred and dissolved to obtain 10mL green mixed solution;
[0068] (2) Add 50 mL of N,N-dimethylformamide to 10 mL of the blue mixed solution obtained in step (1) (the volume after mixing is 60 mL), and place it in a 100 mL polytetrafluoroethylene-lined stainless steel sealed reaction kettle, React at 230°C for 48h, cool, centrifuge, wash the cross-precipitate with ethanol and water for 5 times, and then dry at 100°C for 12h to obtain the precursor powder;
[0069] (3) Mix the precursor powder obtained in step (2) with 0.3964g glucose evenly, then place it in a hydrogen / argon gas mixture (the volume concentration of hydrogen is 5%), sinter at 800°C for 12h, and then After cooling to room temperature, the porous spherical ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com