Double-targeting and pH/oxidation reduction double-sensitive core cross-linking nanoparticle as well as preparation method and application
A nanoparticle and dual-targeting technology, which is applied in the fields of polymer chemistry and biomedical engineering, can solve the problems of poor systemic circulation stability, poor targeting, and poor controlled release effect, and achieve controllable release, good stability, and promote quick release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
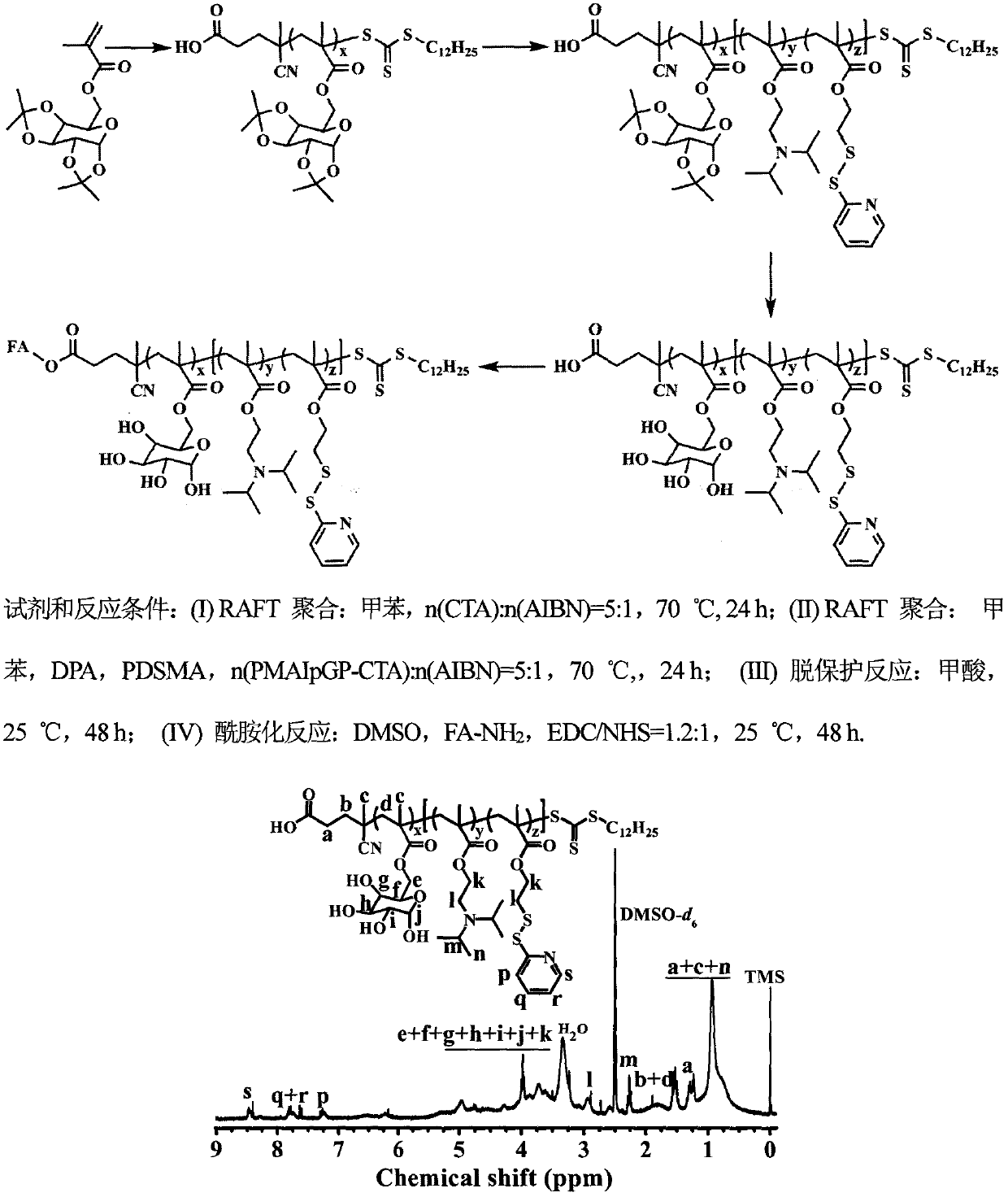
[0054] Example 1: FA-P(MApGP) x -b-P(DPA y -co-PDEMA z )(FA-PMgDP) Synthesis of Amphiphilic Block Polymer
[0055] 1) Preparation of 6-O-methacryloyl chloride-1,2:3,4-O-isopropylidene-D-galactopyranose (MAIpGP) monomer
[0056] Under the condition of ice-water bath, add concentrated sulfuric acid (10mL) dropwise into anhydrous acetone (250mL), dissolve evenly, then add D-(+)-galactose (10g) in batches, stir for 0.5h, then stir at room temperature for 5h . Then use saturated NaOH solution to neutralize the pH of the above mixed solution to 7~8, collect the filtrate by filtration and remove the solvent by rotary evaporation, continue to extract with an appropriate amount of ether to collect the ether phase, and pass through anhydrous MgSO 4 Drying and distillation under reduced pressure gave a yellow syrupy liquid; the yellow syrupy liquid was added to a mixed solution of dry tetrahydrofuran (70mL) and dry triethylamine (7mL), and methacryloyl chloride (6mL) was added to the...
Embodiment 2
[0062] Embodiment 2: Preparation of a series of PMgDP block polymers
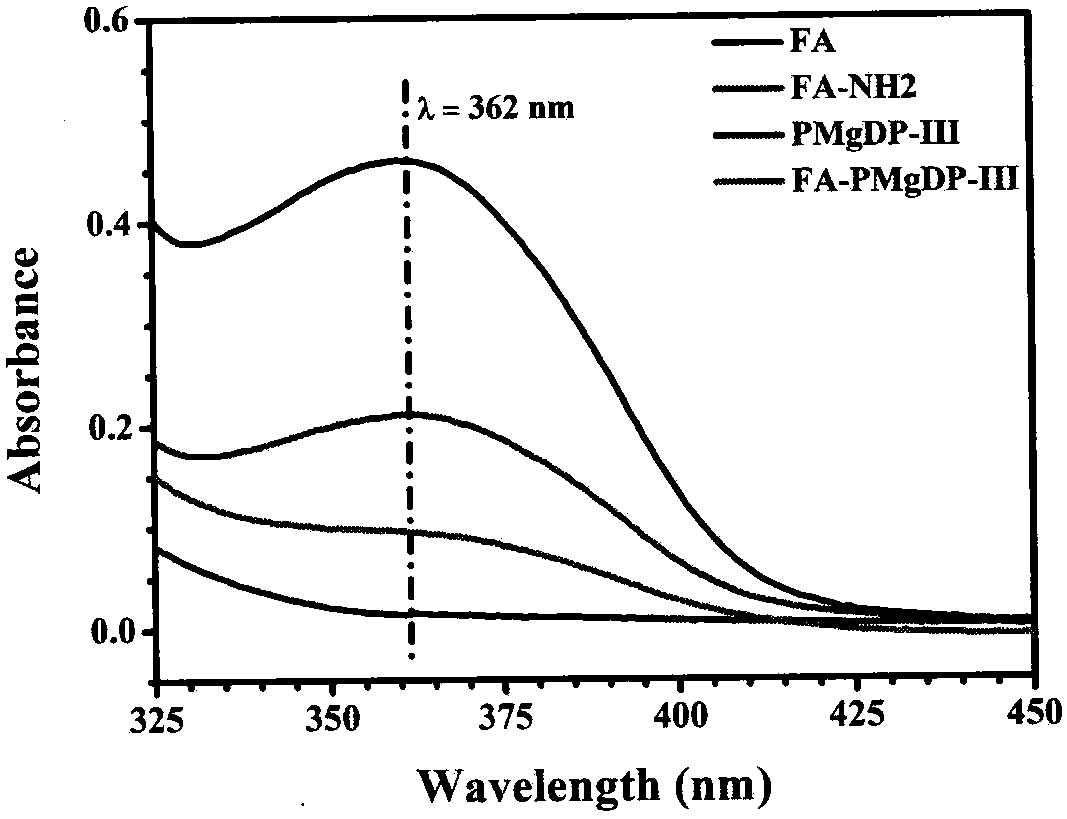
[0063] The device and steps are the same as in Example 1, except that by adjusting the ratio of DPA, PDEMA monomer and macroinitiator PMAIpGP, a series of block polymers PMgDP with different degrees of polymerization and different hydrophilic-hydrophobic ratios are obtained. As shown in Table 1, the PMgDP polymer is characterized by nuclear magnetic resonance spectroscopy, and the preferred results are as follows figure 1 shown. The characteristic peaks of the polymer PMgDP-III obtained in this example were analyzed, and the results showed that the polymer was successfully synthesized, and the composition was consistent with the feed ratio. The FA-PMgDP-III polymer was characterized by ultraviolet spectroscopy, the results are as follows figure 2 shown. Compared with the UV spectrum of PMgDP-III polymer, FA-PMgDP-III polymer appeared the characteristic peak of folic acid at 362nm, indicating that the po...
Embodiment 3
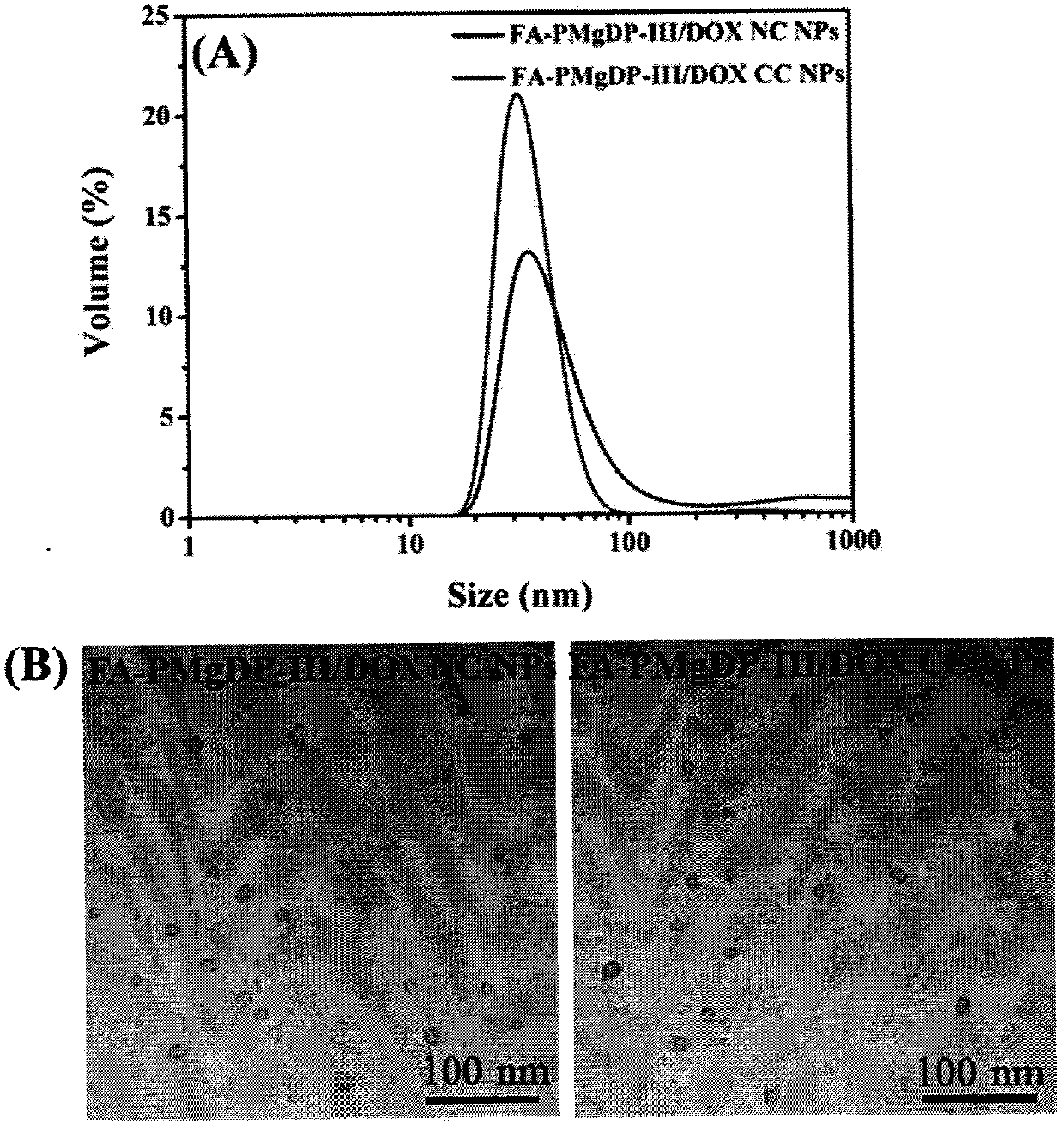
[0067] Embodiment 3: Preparation of Adriamycin-loaded Nanoparticles
[0068] The polymer FA-PMgDP-III and DOX were co-dissolved in DMSO, and five times the amount of PB solution was dropped into the DMSO mixed solution under the condition of magnetic stirring, and then the solution was loaded into a dialysis bag (MWCO: 3500Da) in the PB solution Dialyzed in medium for 24 hours, and the solvent DMSO was removed to obtain a solution of uncrosslinked drug-loaded nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com