Water-free-swallowing taste-masking preparation and preparation method thereof
The technology of a preparation and a taste-masking layer is applied in the field of anhydrous swallowing and taste-masking preparations and the preparation thereof, which can solve the problems of prolonged time of the preparation staying in the mouth, unstable active ingredients, low stability of cefixime, etc. Medication compliance, improving the applicable population, and good taste-masking effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1. Cefixime taste-masking coated pellets
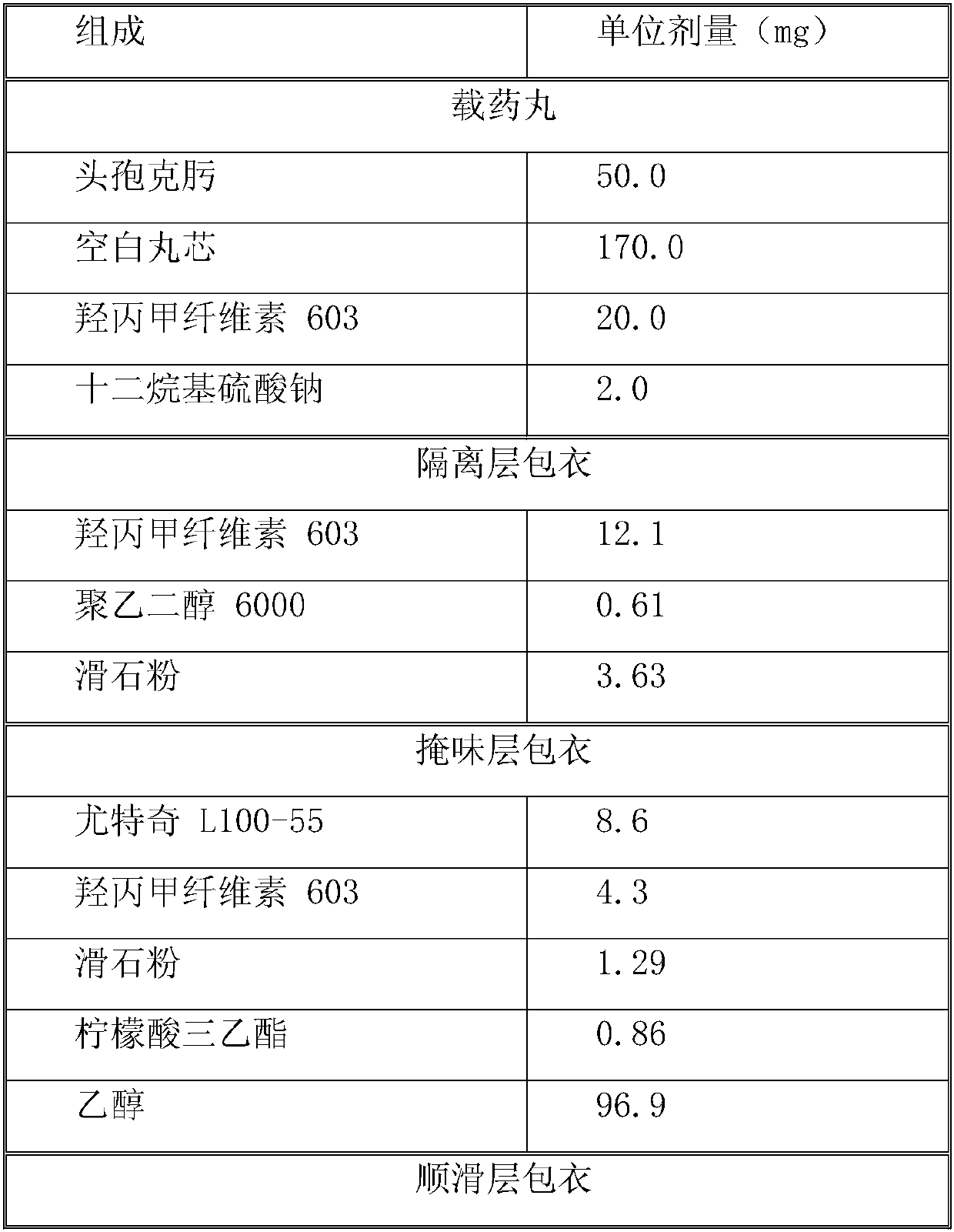
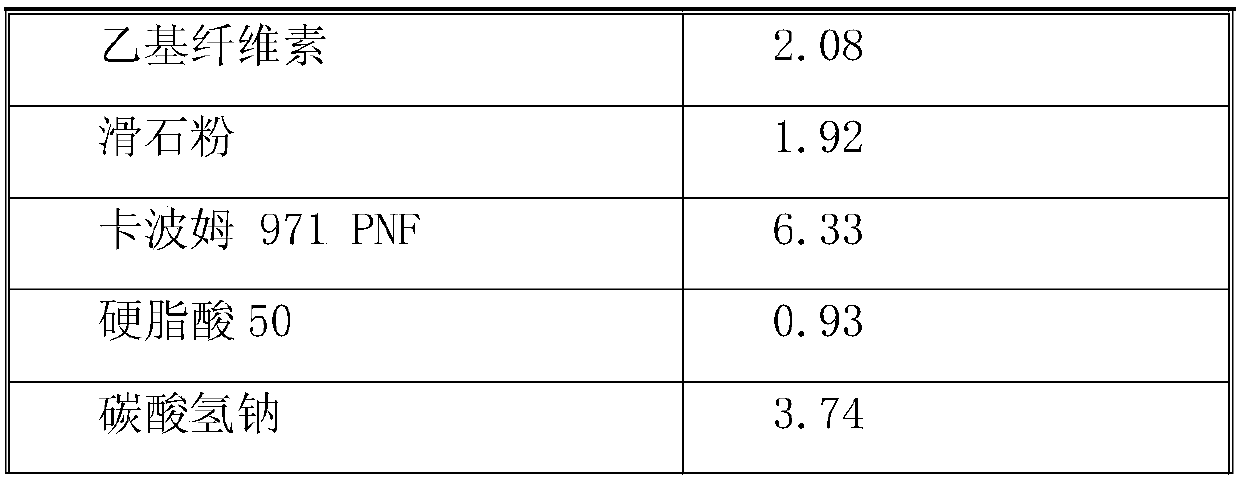
[0031] Cefixime is a macrolide antibiotic with poor drug stability. The active drug cefixime is carried in the form of drug-loaded pellets. Cefixime drug-loaded pellets are coated with an isolation layer, a taste-masking layer, and a smooth layer in sequence. The raw materials of each layer and their dosage are as follows:
[0032]
[0033]
[0034] The preparation method is:
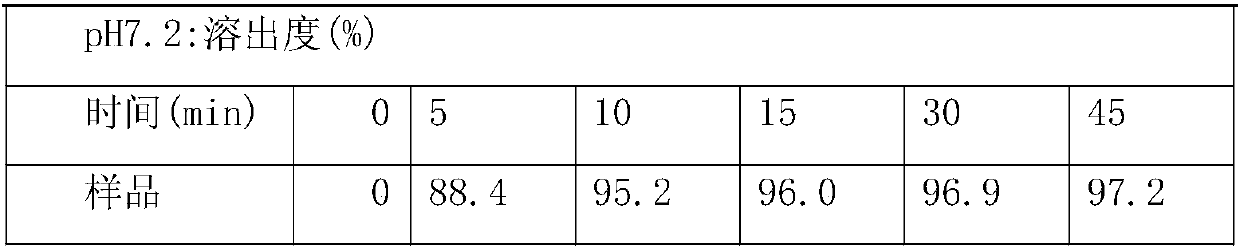
[0035] 1. Preparation of loaded pills: use purified water as solvent, prepare hypromellose 603 into a binder solution, then add the prescribed amount of surfactant sodium lauryl sulfate, add in the dissolved solution while stirring Cefixime, stirred to form a uniform suspension, set aside. Use the 0.5-0.6mm sucrose pellet core as the blank pellet core, and use the fluidized bed bottom spray device to apply the solution. During the application process, the temperature of the material is controlled at 40±5°C. After the application is comple...
Embodiment 2
[0047] Example 2. Clarithromycin taste-masking coated granules
[0048] Use clarithromycin drug powder for direct powder coating, and coat the outer layer of the drug with an isolation layer, a taste-masking layer, and a smooth layer in sequence. The raw materials and dosages of each layer are as follows:
[0049]
[0050]
[0051] The preparation method is:
[0052] 1. Packaging of the isolation layer: according to the prescription dosage, hydroxypropyl cellulose, polyethylene glycol 6000 and magnesium stearate are formulated into a 5% aqueous solution. A fluidized bed is used for the coating of the isolation coat, and the coating process controls the material at 40±5°C.
[0053] 2. Packing of the taste-masking layer: Mix the prescribed amount of ethanol and water to make an alcohol-water solvent, add ethyl cellulose under stirring, add hypromellose 603 until dissolved, stir until dissolved, Add triethyl citrate and talcum powder in turn, stir for 20 minutes to form a...
Embodiment 3
[0063] Example 3. Prednisone taste-masking coated pellets
[0064] The active drug prednisone is loaded in the form of drug-loaded pellets, and the prednisone drug-loaded pellets are sequentially coated with an isolation layer, a taste-masking layer, and a smooth layer. The raw materials and dosage of each layer are as follows:
[0065] Drug-loaded pellets:
[0066]
[0067] 1. Preparation of drug-loaded pellets: Dissolve prednisone, citric acid, hypromellose, and Tween 80 in water to make a solution, select fluidized bed coating, and apply drug coating to the sucrose pellet core. During the coating process, the temperature of the material is controlled at 35-45°C, and the drying is continued for 30 minutes after the coating is completed.
[0068] 2. Isolation layer coating: Hypromellose E5 and talcum powder are prepared into a suspension with water, and fluidized bed coating is selected, and the drug-loaded pellets prepared in the first step are coated with an isolation l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com