Aldehyde-containing metal iridium complexes and their application in the detection and distinction of homocysteine and cysteine
A technology of homocysteine and cysteine, which is applied to compounds containing group 8/9/10/18 elements of the periodic table, material excitation analysis, chemical instruments and methods, etc. Problems such as cystine and cysteine, to achieve the effect of not easy background interference, large Stokes shift, and good phosphorescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] 1. Under nitrogen protection, add 0.3 g (1 mmol) iridium trichloride and 0.4 g (2.5 mmol) 4-(2-pyridyl)-benzaldehyde (Hpba) into a 50 mL two-necked bottle, and then Add 15 mL of deoxygenated ethylene glycol ether / H 2 O (3:1, v / v), reflux for 24 h, cool to room temperature, filter and wash with ethanol several times, then dissolve the solid with a mixed solution of dichloromethane / methanol (1:1, v / v) and depressurize The solvent was spin-dried to obtain an orange-red solid [(Hpba) 2 IrCl] 2 180 mg, yield 55%. 1 HNMR (400MHz, DMSO, MeSi); δ (ppm): 9.88 (d, 2H J=8 Hz), 9.66 (s, 2H), 9.59 (d, 4HJ=8 Hz), 8.48 (d, 2H J=8 Hz), 8.40 (d, 2H J=8 Hz), 8.26 (m, 2H) , 8.17 (m, 2H), 8.04 (m, 4H) ,7.74 (m, 2H), 7.65 (m, 2H), 7.43 (dd, 4H), 6.76 (s, 2H), 6.15 (s, 2H).
[0015] 2. Add 30 mg of [(Hpba) 2 IrCl] 2 with 10 mg of bpy-NH 2 Dissolve in 8 mL of dichloromethane / methanol mixed solution (1:1, v / v), reflux for 12 h under nitrogen protection, cool to room temperature, and di...
Embodiment 2
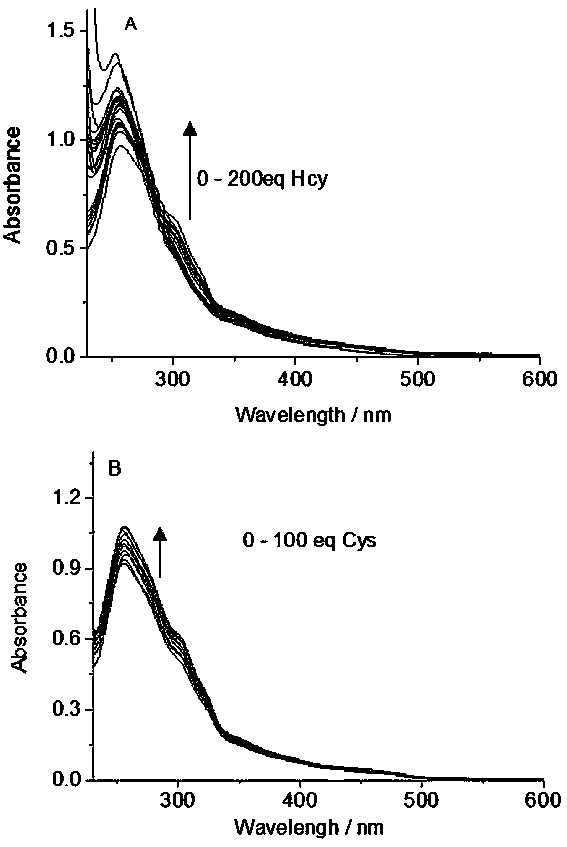
[0017] (1) At a concentration of 2.0×10 -5 Add a series of contents of homocysteine and cysteine to the acetonitrile-Tris buffer solution (4:1, v / v) of the complex Ⅰ in mol / L, and measure the concentration of homocysteine and cysteine at 200-600 UV-Vis absorption spectrum in the nm wavelength range, see the results figure 1 , where A is homocysteine and B is cysteine.
[0018] Such as figure 1 As shown, the absorbance value of complex Ⅰ increases with the increase of amino acid content at the wavelength of 255nm; but the difference between the ultraviolet absorption spectra of homocysteine and cysteine is not significant.
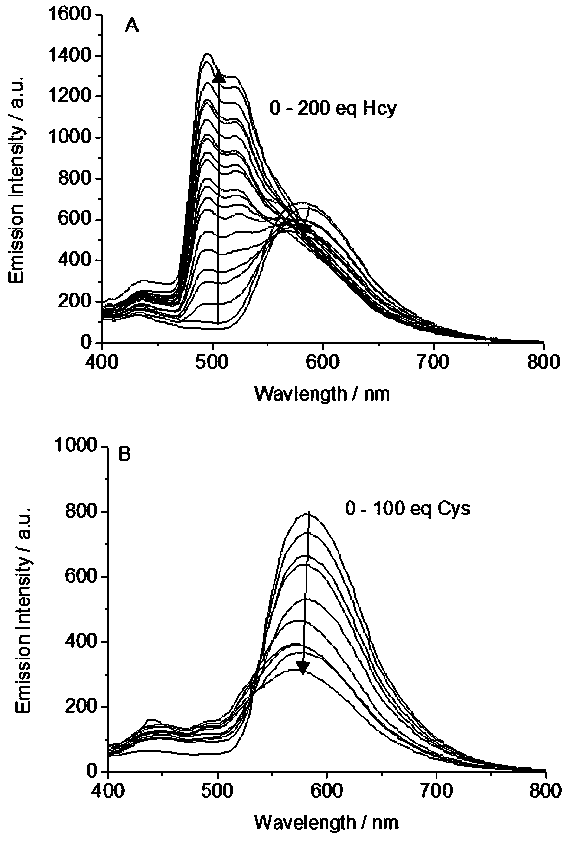
[0019] (2) At a concentration of 2.0×10 -5 A series of homocysteine and cysteine were added to the acetonitrile-Tris buffer solution (4:1, v / v) of the complex Ⅰ in mol / L, and the concentrations of homocysteine and cysteine were respectively determined at 400-800 Fluorescence spectra in the nm wavelength range, see results in fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com