Mn<4+> ion-doped red phosphor, and preparation method and application thereof
A red phosphor, ion doping technology, applied in chemical instruments and methods, luminescent materials, electrical components, etc., can solve the problems of high price, can not meet the application requirements of WLEDs, etc., achieve low cost, good chemical stability and thermal stability. The effect of stability and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
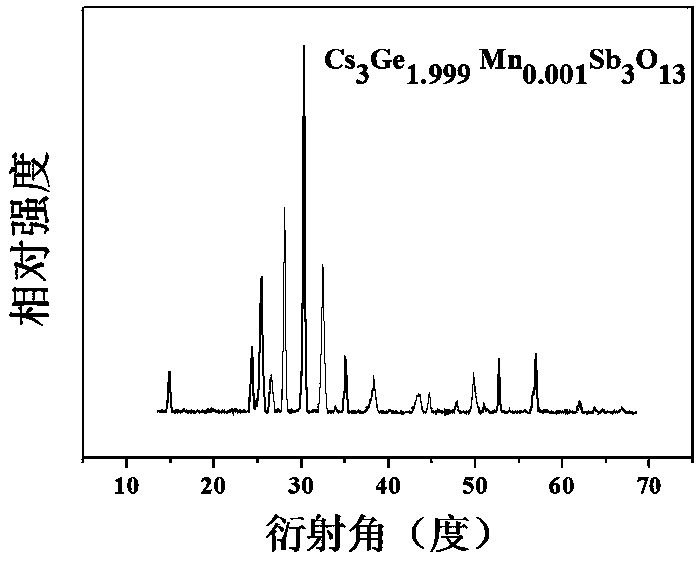
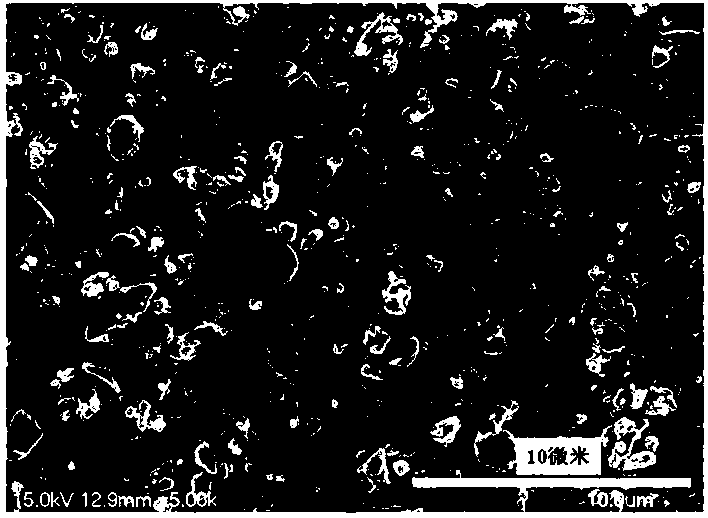
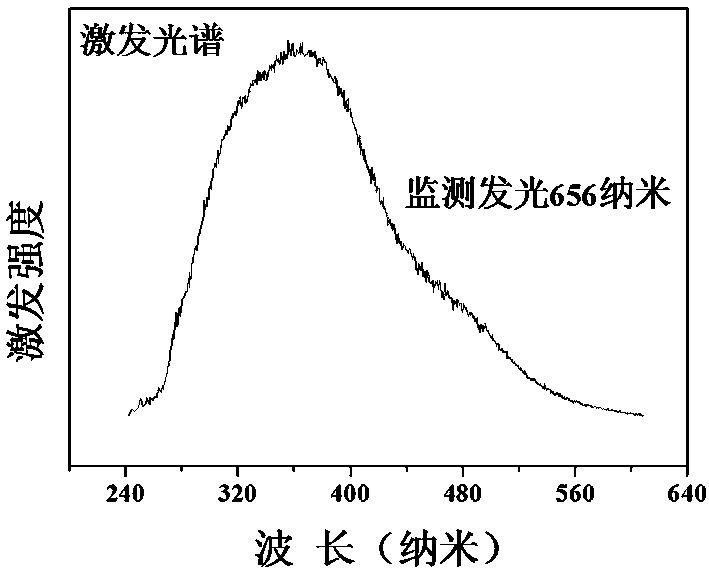
[0034] Preparation of Cs 3 Ge 1.999 mn 0.001 Sb 3 o 13
[0035] According to the chemical formula Cs 3 Ge 1.999 mn 0.001 Sb 3 o 13 , weigh cesium nitrate CsNO respectively according to the stoichiometric ratio 3 : 2.924 g, germanium oxide GeO 2 : 1.045 g; antimony acetate C 6 h 9 o 6 Sb: 4.483 grams; manganese nitrate tetrahydrate Mn (NO 3 ) 2 4H 2 O: 0.001 g; then weigh the corresponding citric acid according to 2.0wt% of the reactant mass in each raw material. Dissolve antimony acetate in an appropriate amount of ethylene glycol solution and stir to obtain solution A; then dissolve the rest of the raw materials in an appropriate amount of dilute nitric acid and stir for 3 hours. After the raw materials, especially germanium oxide, are completely dissolved, the scale Add the obtained citric acid into the above mixture to obtain solution B; slowly add solution A to solution B while stirring, and stir in a water bath at 75°C until it becomes gelatinous. The re...
Embodiment 2
[0043] Preparation of Cs 3 Ge 1.998 mn 0.002 Sb 3 o 13
[0044] According to the chemical formula Cs 3 Ge 1.998 mn 0.002 Sb 3 o 13 , respectively weigh cesium nitrate CsNO 3 : 2.924 grams; germanium tetrachloride GeCl 4 : 2.142 grams (first put appropriate amount of absolute ethanol in a small beaker, peel the balance, then slowly add GeCl 4 , to obtain solution A); antimony acetate C 6 h 9 o 6 Sb: 4.483 grams; manganese acetate Mn (CH 3 COO) 2 : 0.002 gram; Take by weighing corresponding citric acid respectively again by 2.0wt% of reactant quality in each raw material. Dissolve all the weighed citric acid in an appropriate amount of deionized water, then dissolve cesium nitrate and manganese acetate in the citric acid solution to obtain solution B; at the same time, take an appropriate amount of ethylene glycol in another beaker, and weigh the Dissolve antimony acetate to obtain solution C; then slowly add solutions A and C to solution B with a dropper, stir ...
Embodiment 3
[0047] Preparation of Cs 3 Ge 1.994 mn 0.006 Sb 3 o 13
[0048] According to the chemical formula Cs 3 Ge 1.994 mn 0.006 Sb 3 o 13 , respectively weigh cesium acetate C 2 h 3 CaO 2 : 2.879 grams; germanium tetrachloride GeCl 4 : 2.138 grams (first put appropriate amount of absolute ethanol in a small beaker, peel the balance, then slowly add GeCl 4 , to obtain solution A); antimony trichloride SbCl 3 : 3.422 g; Manganese chloride MnCl 2 : 0.005 gram; Then take by weighing corresponding citric acid respectively by 2.0wt% of reactant quality in each raw material. Dissolve all the citric acid taken by weighing in an appropriate amount of deionized water and add 3ml of 37% concentrated hydrochloric acid dropwise, then dissolve cesium acetate, antimony trichloride and manganese chloride in the citric acid solution together to obtain solution B; Then slowly add solution A to solution B with a dropper, and stir while adding. After all solution A is mixed into solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com