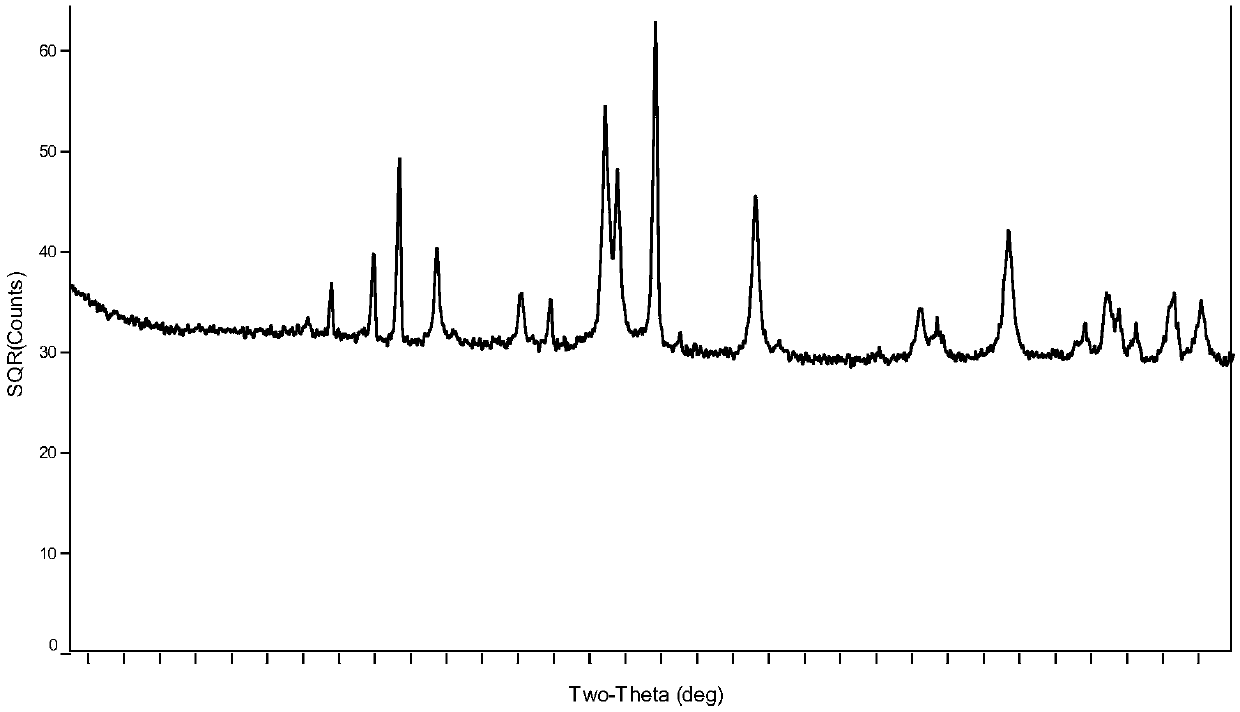
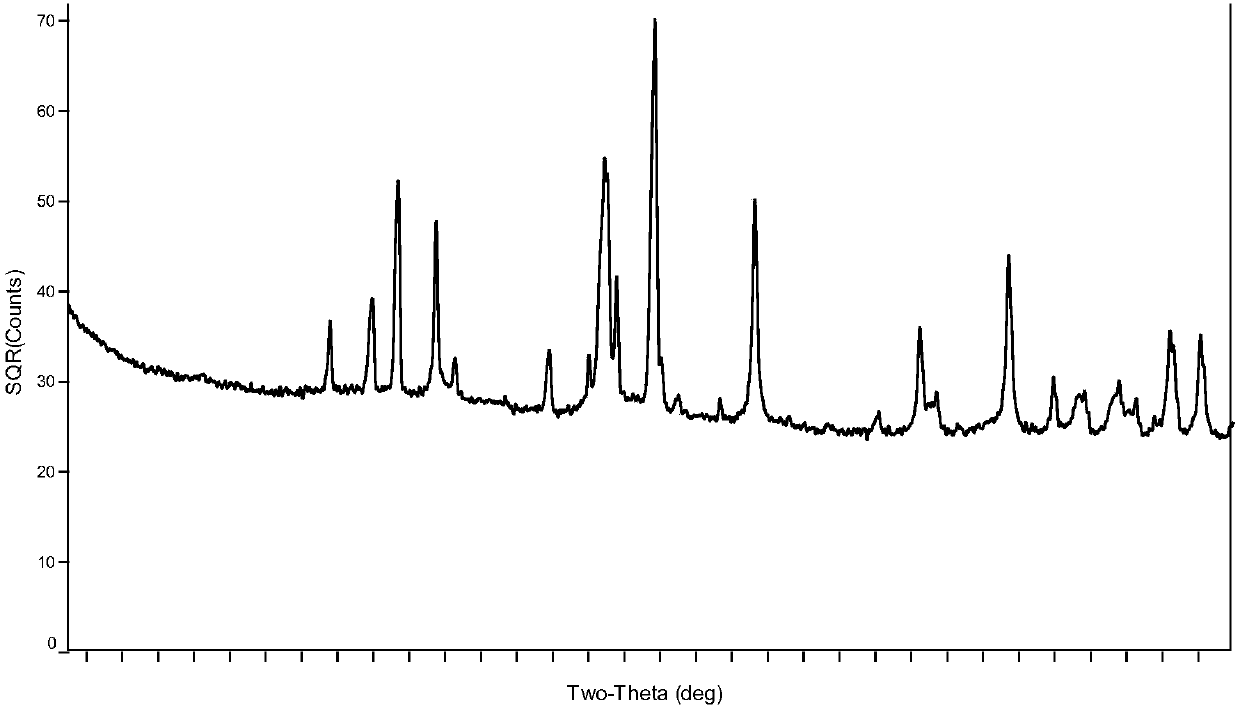
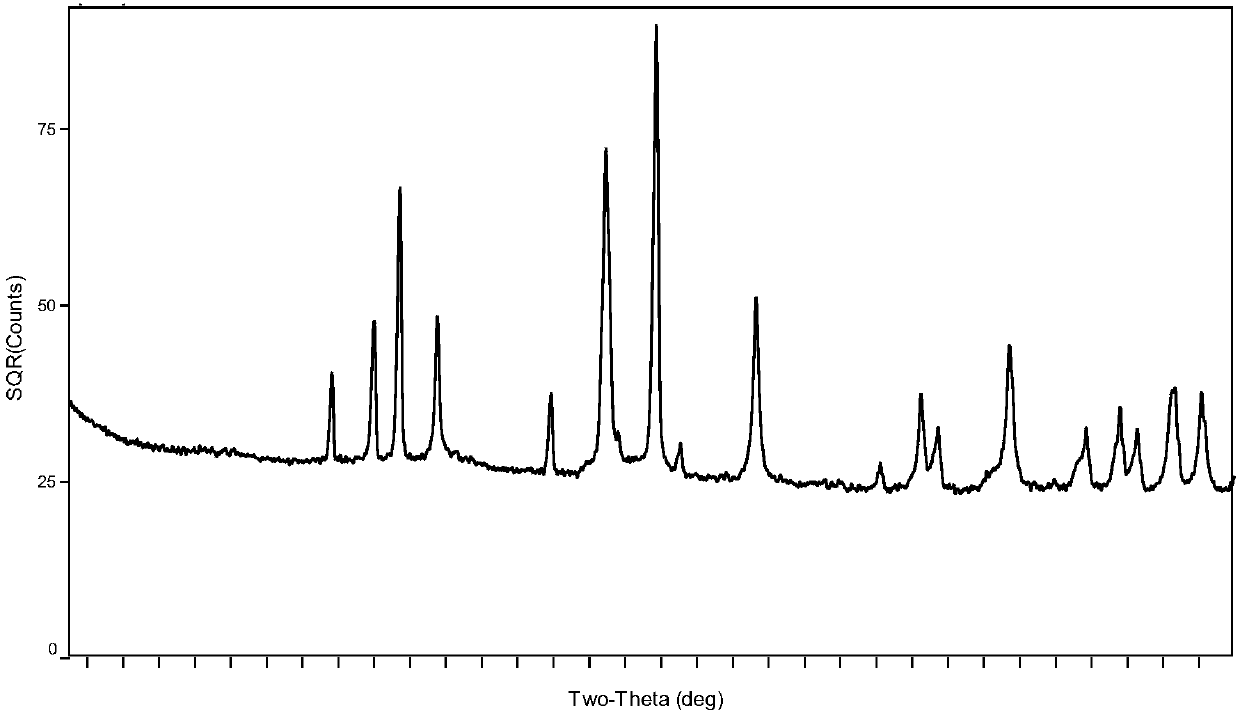
Preparation method of boron carbide superfine powder
A technology of ultra-fine powder and boron carbide, which is applied in chemical instruments and methods, carbon compounds, inorganic chemistry, etc., can solve problems such as difficult to remove, unsuitable for large-scale promotion and development, and achieve labor cost saving, process stability, The effect of prolonging the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The invention discloses a preparation method of boron carbide ultrafine powder, which is based on a boron carbide smelting device.
[0031] Specifically, the boron carbide smelting device includes a smelting furnace body made of refractory bricks and a sunken cover plate made of high-temperature-resistant materials; the smelting furnace body includes a bottom plate and side walls surrounding the bottom plate, smelting There is an opening on the top of the furnace body opposite to the bottom plate, and the sunken cover plate is fastened on the opening; ".
[0032] More specifically, vent holes are opened on the sunken cover plate to remove the gas generated during the smelting process inside the smelting furnace body; positive electrodes and The negative pole, the positive pole and the negative pole all penetrate the inside of the smelting furnace body and the ends of the two are opposite; the inside of the smelting furnace body is filled with smelting raw materials, an...
Embodiment 1
[0052] Ultrafine boron anhydride and ultrafine graphite are used as raw materials, wherein the main content of ultrafine boron anhydride is 99.51%, the average particle size is 8 μm, and the average particle size of ultrafine graphite is 6 μm. 50kg of ultra-fine boron anhydride and 12kg of ultra-fine graphite are fully mixed through a ball mill, and then pressed into strips by an extruder to obtain smelting raw materials.
[0053] The smelting raw materials are placed in a boron carbide smelting device for sintering. The sintering process is as follows: 1000KVA transformer (rectifier), 15 gears of on-load tap changers, and power transmission in series. Starting from gear 1, the gears are gradually increased. Observe For the rise of the primary and secondary currents, adjust the interval between upshifts as appropriate. Whenever the primary current reaches 70A, downgrade by one gear. According to the furnace resistance and voltage conditions, adjust to parallel connection and en...
Embodiment 2
[0059] Recovered boric anhydride (dust collected in electric arc smelting), domestic ultra-fine boron oxide, ultra-fine graphite and ultra-fine carbon powder are used as raw materials, and the recovered boron anhydride is pretreated first, including removal of abnormally large particles and impurities, to obtain the main The content is 98.01%, and the average particle size is 12 μm ultrafine boric anhydride, and the particle size of the rest of the raw materials are all within 10 μm. Stir and mix 40kg ultrafine boric anhydride, 10kg domestic ultrafine boron oxide, 8kg ultrafine graphite and 4kg ultrafine carbon powder, grind them with a ball mill and press them into 8cm-12cm long squares with a compacted density of about 1.24g / cm 3 , to obtain smelting raw materials.
[0060] The smelting raw materials are placed in a boron carbide smelting device for sintering. The sintering process is as follows: 1000KVA transformer (rectifier), 15 gears of on-load tap changers, and power t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com